Initial FGF23-mediated signaling occurs in the distal convoluted tubule - PubMed (original) (raw)
Initial FGF23-mediated signaling occurs in the distal convoluted tubule
Emily G Farrow et al. J Am Soc Nephrol. 2009 May.
Abstract
Fibroblast growth factor-23 (FGF23), a hormone central to phosphate and vitamin D metabolism, reduces renal absorption of phosphate by downregulating the sodium-phosphate cotransporter Npt2a. However, the mechanisms of FGF23 action in the kidney are unclear, as Npt2a localizes to the proximal tubule (PT) and the FGF23 coreceptor alpha-Klotho (KL) localizes to the distal convoluted tubule (DCT). Immunofluorescent analyses following FGF23 injection in mice showed robust staining for phospho-ERK1/2, a marker of FGF23 bioactivity, only within the DCT in a subset of KL-positive cells. This activity colocalized with the FGF23 receptor FGFR1 and was present in DCT cells that were adjacent to Npt2a-expressing PT segments. Although KL is expressed as both secreted and membrane-bound isoforms, only the membrane-bound isoform was capable of mediating FGF23 bioactivity. These findings provide novel insight into the mechanisms of hormone-regulated phosphate metabolism by identifying an intrarenal signaling axis for FGF23.
Figures
Figure 1.
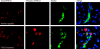
FGF23-dependent p-ERK1/2 signaling in kidney. Results from vehicle-injected animals are shown in the upper panels, and FGF23-injected animals in the lower panels at 5 min postinjection (10-min results were identical). Total ERK1/2 staining was equally positive in all nephron segments for vehicle- and FGF23-injected animals. Phospho-ERK1/2 staining was only observed in the FGF23-injected animals; KL was positive in both vehicle- and FGF23-injected animals. p-ERK1/2 staining (red) localized to the nucleus in the same nephron segment as KL (green) in FGF23-injected mice, as shown by p-ERK1/2 and KL costaining (Merge column; arrows show positive nuclear p-ERK1/2 colocalized with KL). Nuclei were stained blue using DAPI in the Merge column.
Figure 2.
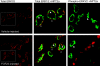
Distribution of FGF23 activity and Npt2a. Total ERK1/2 (red) was detected widely throughout the kidney (left panels; low magnification). Total ERK1/2 (red, open arrowheads) colocalized with Npt2a (green, closed arrowhead) in the PT (boxed area from left panels are shown at high magnification in the center panels). Npt2a was detected in vehicle-injected animals; however, p-ERK1/2 staining was not observed in control mice (right panel, open arrowhead). In contrast, p-ERK1/2 staining (red) was readily detectable and spatially separated from Npt2a in the PT (green, closed arrowhead) in the FGF23-injected mice (right panel).
Figure 3.
Localization of p-ERK1/2 and FGFR1. FGFR1 (red) staining was detected in the kidney (left panel). Staining for p-ERK1/2 (green, center panel) colocalized with a subset of FGFR1-positive cells in FGF23-injected mice. Arrows highlight cells that are FGFR1 and p-ERK1/2 positive in the same kidney section.
Figure 4.
FGF23-dependent KL activity. (A) Full-length mKL was detected in the lysate (Lys), but not in the cell media (Med) using an anti-V5 antibody to a C-terminal tag (left panels). cKL was detectable in the cell media using an anti-human KL antibody to an extracellular KL epitope (center). sKL was detected in the cell lysate and media using anti-V5 (right). (B) HEK293 cells stably expressing mKL or sKL, and native HEK293 cells treated with cKL were treated with vehicle or 5, 10, 50, and 100 ng/ml of FGF23 for 30 min. EGR1 mRNA, assessed by quantitative PCR, showed a dose-dependent increase in the mKL cells (*P < 0.05). There was no difference in EGR1 mRNA compared with control in either sKL or cKL. (Inset) Western blot shows one-half of the total mKL, cKL, and sKL protein present per well for the qPCR experiments graphed in Figure 4B. (C) A time-dependent increase in EGR1 mRNA compared with control was observed when mKL cells were treated with 100 ng/ml of FGF23 for 10, 20, and 30 min (*P < 0.05). (D) HEK293 cells stably expressing mKL or sKL, and native HEK293 cells treated with cKL, were treated with 100 ng/ml of FGF23 for 0, 5, and 15 min. p-ERK1/2 expression, as assessed by Western blot analyses, was only detected in the mKL cells (upper panel). Total ERK1/2 was similar for all cells across treatments (lower panel).
Similar articles
- Altered renal FGF23-mediated activity involving MAPK and Wnt: effects of the Hyp mutation.
Farrow EG, Summers LJ, Schiavi SC, McCormick JA, Ellison DH, White KE. Farrow EG, et al. J Endocrinol. 2010 Oct;207(1):67-75. doi: 10.1677/JOE-10-0181. Epub 2010 Jul 30. J Endocrinol. 2010. PMID: 20675303 Free PMC article. - [Discovery of alpha-Klotho and FGF23 unveiled new insight into calcium and phosphate homeostasis].
Nabeshima Y. Nabeshima Y. Clin Calcium. 2008 Jul;18(7):923-34. Clin Calcium. 2008. PMID: 18591743 Review. Japanese. - Convergent Signaling Pathways Regulate Parathyroid Hormone and Fibroblast Growth Factor-23 Action on NPT2A-mediated Phosphate Transport.
Sneddon WB, Ruiz GW, Gallo LI, Xiao K, Zhang Q, Rbaibi Y, Weisz OA, Apodaca GL, Friedman PA. Sneddon WB, et al. J Biol Chem. 2016 Sep 2;291(36):18632-42. doi: 10.1074/jbc.M116.744052. Epub 2016 Jul 18. J Biol Chem. 2016. PMID: 27432882 Free PMC article. - Regulation of renal phosphate transport by FGF23 is mediated by FGFR1 and FGFR4.
Gattineni J, Alphonse P, Zhang Q, Mathews N, Bates CM, Baum M. Gattineni J, et al. Am J Physiol Renal Physiol. 2014 Feb 1;306(3):F351-8. doi: 10.1152/ajprenal.00232.2013. Epub 2013 Nov 20. Am J Physiol Renal Physiol. 2014. PMID: 24259513 Free PMC article. - Pleiotropic Actions of FGF23.
Erben RG. Erben RG. Toxicol Pathol. 2017 Oct;45(7):904-910. doi: 10.1177/0192623317737469. Epub 2017 Nov 2. Toxicol Pathol. 2017. PMID: 29096595 Free PMC article. Review.
Cited by
- The Effect of Periodontitis on Fibroblast Growth Factor 23 Levels in Predialysis Chronic Kidney Disease Patients.
Wan Abdul Azim WA, Kassim NK, Taib H, Abdullah NH, Che Abdul Aziz NA, Ibrahim HA. Wan Abdul Azim WA, et al. Cureus. 2024 Jul 23;16(7):e65166. doi: 10.7759/cureus.65166. eCollection 2024 Jul. Cureus. 2024. PMID: 39176315 Free PMC article. - Determination of the reference interval for urinary klotho to creatinine ratio of healthy dogs.
Marečáková N, Kačírová J, Tóthová C, Maďari A, Maďar M, Farbáková J, Horňák S. Marečáková N, et al. Front Vet Sci. 2024 Jul 23;11:1423390. doi: 10.3389/fvets.2024.1423390. eCollection 2024. Front Vet Sci. 2024. PMID: 39113723 Free PMC article. - The Intricacies of Renal Phosphate Reabsorption-An Overview.
Walker V. Walker V. Int J Mol Sci. 2024 Apr 25;25(9):4684. doi: 10.3390/ijms25094684. Int J Mol Sci. 2024. PMID: 38731904 Free PMC article. Review. - Diagnosis and management of tumor-induced osteomalacia: a single center experience.
Hacisahinogullari H, Tekin S, Tanrikulu S, Saribeyliler G, Yalin GY, Bilgic B, Isik EG, Salduz A, Tuncer S, Gul N, Uzum AK, Aral F, Tanakol R, Selcukbiricik OS. Hacisahinogullari H, et al. Endocrine. 2023 Nov;82(2):427-434. doi: 10.1007/s12020-023-03450-3. Epub 2023 Jul 22. Endocrine. 2023. PMID: 37480497 - Fibroblast Growth Factor 23 Bone Regulation and Downstream Hormonal Activity.
Clinkenbeard E. Clinkenbeard E. Calcif Tissue Int. 2023 Jul;113(1):4-20. doi: 10.1007/s00223-023-01092-1. Epub 2023 Jun 12. Calcif Tissue Int. 2023. PMID: 37306735 Review.
References
- ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26: 345–348, 2000 - PubMed
- Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H: Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348: 1656–1663, 2003 - PubMed
- Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB: Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145: 3087–3094, 2004 - PubMed
- Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T: FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun 314: 409–414, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous