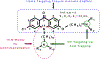Discovery of dual function acridones as a new antimalarial chemotype - PubMed (original) (raw)
. 2009 May 14;459(7244):270-3.
doi: 10.1038/nature07937. Epub 2009 Apr 8.
Martin J Smilkstein, Reto Brun, Sergio Wittlin, Roland A Cooper, Kristin D Lane, Aaron Janowsky, Robert A Johnson, Rozalia A Dodean, Rolf Winter, David J Hinrichs, Michael K Riscoe
Affiliations
- PMID: 19357645
- PMCID: PMC8158239
- DOI: 10.1038/nature07937
Discovery of dual function acridones as a new antimalarial chemotype
Jane X Kelly et al. Nature. 2009.
Abstract
Preventing and delaying the emergence of drug resistance is an essential goal of antimalarial drug development. Monotherapy and highly mutable drug targets have each facilitated resistance, and both are undesirable in effective long-term strategies against multi-drug-resistant malaria. Haem remains an immutable and vulnerable target, because it is not parasite-encoded and its detoxification during haemoglobin degradation, critical to parasite survival, can be subverted by drug-haem interaction as in the case of quinolines and many other drugs. Here we describe a new antimalarial chemotype that combines the haem-targeting character of acridones, together with a chemosensitizing component that counteracts resistance to quinoline antimalarial drugs. Beyond the essential intrinsic characteristics common to deserving candidate antimalarials (high potency in vitro against pan-sensitive and multi-drug-resistant Plasmodium falciparum, efficacy and safety in vivo after oral administration, inexpensive synthesis and favourable physicochemical properties), our initial lead, T3.5 (3-chloro-6-(2-diethylamino-ethoxy)-10-(2-diethylamino-ethyl)-acridone), demonstrates unique synergistic properties. In addition to 'verapamil-like' chemosensitization to chloroquine and amodiaquine against quinoline-resistant parasites, T3.5 also results in an apparently mechanistically distinct synergism with quinine and with piperaquine. This synergy, evident in both quinoline-sensitive and quinoline-resistant parasites, has been demonstrated both in vitro and in vivo. In summary, this innovative acridone design merges intrinsic potency and resistance-counteracting functions in one molecule, and represents a new strategy to expand, enhance and sustain effective antimalarial drug combinations.
Conflict of interest statement
Competing Interests statement The authors declare that they have no competing financial interests.
Figures
Figure 1.
Generalized chemical structure of dual-function acridone derivatives. The rigid tricyclic aromatic acridone core promotes π-π stacking for heme binding. The side chain attachment at the central nitrogen atom provides a hydrogen bond acceptor needed for the chemosensitization function, and together with the side chain at the 6-position facilitate accumulation in the digestive vacuole (DV) via acid trapping.
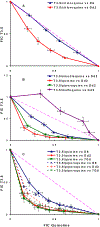
Figure 2.
Isobolograms of the in vitro interaction of A) T3.5/chloroquine against MDR P. falciparum strain Dd2 and chloroquine sensitive P. falciparum strain D6 (mean FIC indices are 0.72 and 0.97, respectively); B) T3.5/amodiaquine, T3.5/mefloquine, T3.5/quinine and T3.5/piperaquine combinations against MDR strain Dd2 (mean FIC indices are 0.73, 1.25, 0.50, and 0.60, respectively); C) T3.5/quinine (solid line) combination against chloroquine sensitive strain D6 and MDR strains Dd2 and 7G8 (mean FIC indices are 0.64, 0.50, and 0.49, respectively); T3.5/piperaquine (dashed line) combination against chloroquine sensitive strain D6 and MDR strains Dd2 and 7G8 (mean FIC indices are 0.66, 0.60, and 0.51, respectively). The mean FIC indices ± standard errors of the mean (S.E.M.) were derived from three independent experiments. The _x_-axis represents the FICs of quinolines, and _y_-axis represents the FICs of T3.5. The diagonal line (FIC index = 1) indicates the hypothetical additive drug effect. A concave curve (FIC index < 1) below the diagonal line typically indicates synergy of the combination, while a convex curve (FIC index > 1) above the diagonal line indicates antagonism.
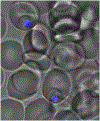
Figure 3.
Confocal microscopy of localized T3.5 fluorescence in two intraerythrocytic P. falciparum trophozoites. Fluorescence was determined in live _P. falciparum_-infected erythrocytes using a laser (emission line 351 nm) scanning confocal microscope. The figure shows the intrinsic fluorescence of T3.5 (blue) superimposed on the brightfield transmission image of the infected cells.
Similar articles
- Design, synthesis, and evaluation of 10-N-substituted acridones as novel chemosensitizers in Plasmodium falciparum.
Kelly JX, Smilkstein MJ, Cooper RA, Lane KD, Johnson RA, Janowsky A, Dodean RA, Hinrichs DJ, Winter R, Riscoe M. Kelly JX, et al. Antimicrob Agents Chemother. 2007 Nov;51(11):4133-40. doi: 10.1128/AAC.00669-07. Epub 2007 Sep 10. Antimicrob Agents Chemother. 2007. PMID: 17846138 Free PMC article. - Inhibition of efflux of quinolines as new therapeutic strategy in malaria.
Henry M, Alibert S, Rogier C, Barbe J, Pradines B. Henry M, et al. Curr Top Med Chem. 2008;8(7):563-78. doi: 10.2174/156802608783955593. Curr Top Med Chem. 2008. PMID: 18473883 Review. - A Variant PfCRT Isoform Can Contribute to Plasmodium falciparum Resistance to the First-Line Partner Drug Piperaquine.
Dhingra SK, Redhi D, Combrinck JM, Yeo T, Okombo J, Henrich PP, Cowell AN, Gupta P, Stegman ML, Hoke JM, Cooper RA, Winzeler E, Mok S, Egan TJ, Fidock DA. Dhingra SK, et al. mBio. 2017 May 9;8(3):e00303-17. doi: 10.1128/mBio.00303-17. mBio. 2017. PMID: 28487425 Free PMC article. - A 2-amino quinoline, 5-(3-(2-(7-chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid, interacts with PfMDR1 and inhibits its drug transport in Plasmodium falciparum.
Edaye S, Reiling SJ, Leimanis ML, Wunderlich J, Rohrbach P, Georges E. Edaye S, et al. Mol Biochem Parasitol. 2014 Jun;195(1):34-42. doi: 10.1016/j.molbiopara.2014.05.006. Epub 2014 Jun 8. Mol Biochem Parasitol. 2014. PMID: 24914817 - Quinoline drug-heme interactions and implications for antimalarial cytostatic versus cytocidal activities.
Gorka AP, de Dios A, Roepe PD. Gorka AP, et al. J Med Chem. 2013 Jul 11;56(13):5231-46. doi: 10.1021/jm400282d. Epub 2013 Apr 29. J Med Chem. 2013. PMID: 23586757 Review.
Cited by
- Recent advances in the discovery of haem-targeting drugs for malaria and schistosomiasis.
de Villiers KA, Egan TJ. de Villiers KA, et al. Molecules. 2009 Aug 4;14(8):2868-87. doi: 10.3390/molecules14082868. Molecules. 2009. PMID: 19701131 Free PMC article. Review. - Acridones Are Highly Potent Inhibitors of Toxoplasma gondii Tachyzoites.
Alday PH, McConnell EV, Boitz Zarella JM, Dodean RA, Kancharla P, Kelly JX, Doggett JS. Alday PH, et al. ACS Infect Dis. 2021 Jul 9;7(7):1877-1884. doi: 10.1021/acsinfecdis.1c00016. Epub 2021 Mar 16. ACS Infect Dis. 2021. PMID: 33723998 Free PMC article. - High-throughput matrix screening identifies synergistic and antagonistic antimalarial drug combinations.
Mott BT, Eastman RT, Guha R, Sherlach KS, Siriwardana A, Shinn P, McKnight C, Michael S, Lacerda-Queiroz N, Patel PR, Khine P, Sun H, Kasbekar M, Aghdam N, Fontaine SD, Liu D, Mierzwa T, Mathews-Griner LA, Ferrer M, Renslo AR, Inglese J, Yuan J, Roepe PD, Su XZ, Thomas CJ. Mott BT, et al. Sci Rep. 2015 Sep 25;5:13891. doi: 10.1038/srep13891. Sci Rep. 2015. PMID: 26403635 Free PMC article. - Sequential catalysis: exploiting a single rhodium(i) catalyst to promote an alkyne hydroacylation-aryl boronic acid conjugate addition sequence.
Fernández M, Castaing M, Willis MC. Fernández M, et al. Chem Sci. 2017 Jan 1;8(1):536-540. doi: 10.1039/c6sc03066a. Epub 2016 Sep 9. Chem Sci. 2017. PMID: 28451201 Free PMC article. - PfCRT-mediated drug transport in malarial parasites.
Roepe PD. Roepe PD. Biochemistry. 2011 Jan 18;50(2):163-71. doi: 10.1021/bi101638n. Epub 2010 Dec 22. Biochemistry. 2011. PMID: 21142008 Free PMC article. Review.
References
- Olliaro PL & Goldberg DE The Plasmodium digestive Vacuole: Metabolic Headquarters and Choice Drug Target. Parasitol Today 11, 294–7 (1995). - PubMed
- Wellems TE Plasmodium chloroquine resistance and the search for a replacement antimalarial drug. Science 298, 124–6 (2002). - PubMed
- Kumar S, Guha M, Choubey V, Maity P & Bandyopadhyay U Antimalarial drugs inhibiting hemozoin (beta-hematin) formation: a mechanistic update. Life Sci 80, 813–28 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources