Hemoglobin cleavage site-specificity of the Plasmodium falciparum cysteine proteases falcipain-2 and falcipain-3 - PubMed (original) (raw)
Hemoglobin cleavage site-specificity of the Plasmodium falciparum cysteine proteases falcipain-2 and falcipain-3
Shoba Subramanian et al. PLoS One. 2009.
Abstract
The Plasmodium falciparum cysteine proteases falcipain-2 and falcipain-3 degrade host hemoglobin to provide free amino acids for parasite protein synthesis. Hemoglobin hydrolysis has been described as an ordered process initiated by aspartic proteases, but cysteine protease inhibitors completely block the process, suggesting that cysteine proteases can also initiate hemoglobin hydrolysis. To characterize the specific roles of falcipains, we used three approaches. First, using random P(1) - P(4) amino acid substrate libraries, falcipain-2 and falcipain-3 demonstrated strong preference for cleavage sites with Leu at the P(2) position. Second, with overlapping peptides spanning alpha and beta globin and proteolysis-dependent (18)O labeling, hydrolysis was seen at many cleavage sites. Third, with intact hemoglobin, numerous cleavage products were identified. Our results suggest that hemoglobin hydrolysis by malaria parasites is not a highly ordered process, but rather proceeds with rapid cleavage by falcipains at multiple sites. However, falcipain-2 and falcipain-3 show strong specificity for P(2) Leu in small peptide substrates, in agreement with the specificity in optimized small molecule inhibitors that was identified previously. These results are consistent with a principal role of falcipain-2 and falcipain-3 in the hydrolysis of hemoglobin by P. falciparum and with the possibility of developing small molecule inhibitors with optimized specificity as antimalarial agents.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
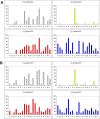
Figure 1. Cleavage preferences of falcipain-2 and falcipain-3 against a tetrapeptide library.
P1 P2, P3 and P4 complete diverse libraries were used to determine specificities. Activities are displayed as percentage of the maximum for each position. Amino acids are represented by the single-letter code (n is for norleucine). Error bars represent standard deviations of results from three experiments.
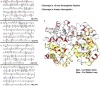
Figure 2. Hemoglobin cleavage sites for falcipain-2 and falcipain-3.
A–D. Globin peptides and intact human hemoglobin. Cleavage sites for the two proteases based on analysis as described in Methods of hydrolysis of 12-mer peptides (top arrows, data from all time points) and intact hemoglobin (lower arrows, data from both 180 min and overnight time points) are shown for α and β globin. E. Intact human hemoglobin. The schematic shows α globin subunits in white and β globin subunits in yellow. P1 residues at cleavage sites within helices are in red and within loops are in blue.
Figure 3. Time course for cleavage of globin peptides.
Schematic representation of cleavage fragments over the indicated time points based on analysis of cleavage of 12-mer peptides spanning the sequences of α and β globin.
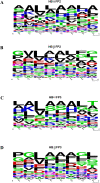
Figure 4. Cleavage site preferences for falcipain-2 and falcipain-3.
Preferences for amino acids (single letter code) at P1-P4 and P1′-P4′ are shown schematically. At each position, the height of each amino acid corresponds to its frequency at cleavage sites based on analysis of α and β globin. WebLogo (
weblogo.berkeley.edu
), a free software for plotting consensus sequences, was used to plot P4- P4′ preferences.
Similar articles
- Expression and characterization of the Plasmodium falciparum haemoglobinase falcipain-3.
Sijwali PS, Shenai BR, Gut J, Singh A, Rosenthal PJ. Sijwali PS, et al. Biochem J. 2001 Dec 1;360(Pt 2):481-9. doi: 10.1042/0264-6021:3600481. Biochem J. 2001. PMID: 11716777 Free PMC article. - Gene disruption confirms a critical role for the cysteine protease falcipain-2 in hemoglobin hydrolysis by Plasmodium falciparum.
Sijwali PS, Rosenthal PJ. Sijwali PS, et al. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4384-9. doi: 10.1073/pnas.0307720101. Epub 2004 Mar 15. Proc Natl Acad Sci U S A. 2004. PMID: 15070727 Free PMC article. - Characterization of native and recombinant falcipain-2, a principal trophozoite cysteine protease and essential hemoglobinase of Plasmodium falciparum.
Shenai BR, Sijwali PS, Singh A, Rosenthal PJ. Shenai BR, et al. J Biol Chem. 2000 Sep 15;275(37):29000-10. doi: 10.1074/jbc.M004459200. J Biol Chem. 2000. PMID: 10887194 - Cysteine proteases of malaria parasites.
Rosenthal PJ. Rosenthal PJ. Int J Parasitol. 2004 Dec;34(13-14):1489-99. doi: 10.1016/j.ijpara.2004.10.003. Int J Parasitol. 2004. PMID: 15582526 Review. - Falcipain cysteine proteases of malaria parasites: An update.
Rosenthal PJ. Rosenthal PJ. Biochim Biophys Acta Proteins Proteom. 2020 Mar;1868(3):140362. doi: 10.1016/j.bbapap.2020.140362. Epub 2020 Jan 9. Biochim Biophys Acta Proteins Proteom. 2020. PMID: 31927030 Review.
Cited by
- Allosteric pockets and dynamic residue network hubs of falcipain 2 in mutations including those linked to artemisinin resistance.
Okeke CJ, Musyoka TM, Sheik Amamuddy O, Barozi V, Tastan Bishop Ö. Okeke CJ, et al. Comput Struct Biotechnol J. 2021 Oct 8;19:5647-5666. doi: 10.1016/j.csbj.2021.10.011. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34745456 Free PMC article. - Cysteine Proteases: Modes of Activation and Future Prospects as Pharmacological Targets.
Verma S, Dixit R, Pandey KC. Verma S, et al. Front Pharmacol. 2016 Apr 25;7:107. doi: 10.3389/fphar.2016.00107. eCollection 2016. Front Pharmacol. 2016. PMID: 27199750 Free PMC article. Review. - Protease-associated cellular networks in malaria parasite Plasmodium falciparum.
Lilburn TG, Cai H, Zhou Z, Wang Y. Lilburn TG, et al. BMC Genomics. 2011 Dec 23;12 Suppl 5(Suppl 5):S9. doi: 10.1186/1471-2164-12-S5-S9. Epub 2011 Dec 23. BMC Genomics. 2011. PMID: 22369208 Free PMC article. - South African Abietane Diterpenoids and Their Analogs as Potential Antimalarials: Novel Insights from Hybrid Computational Approaches.
Musyoka T, Bishop ÖT. Musyoka T, et al. Molecules. 2019 Nov 7;24(22):4036. doi: 10.3390/molecules24224036. Molecules. 2019. PMID: 31703388 Free PMC article. - Biochemical properties of a novel cysteine protease of Plasmodium vivax, vivapain-4.
Na BK, Bae YA, Zo YG, Choe Y, Kim SH, Desai PV, Avery MA, Craik CS, Kim TS, Rosenthal PJ, Kong Y. Na BK, et al. PLoS Negl Trop Dis. 2010 Oct 12;4(10):e849. doi: 10.1371/journal.pntd.0000849. PLoS Negl Trop Dis. 2010. PMID: 20967286 Free PMC article.
References
- Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64:1–11. - PubMed
- Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–218. - PubMed
- Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77:181–192. - PubMed
- Alker AP, Lim P, Sem R, Shah NK, Yi P, et al. Pfmdr1 and in vivo resistance to artesunate-mefloquine in falciparum malaria on the Cambodian-Thai border. Am J Trop Med Hyg. 2007;76:641–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI035707/AI/NIAID NIH HHS/United States
- R56 AI035800/AI/NIAID NIH HHS/United States
- R29 AI035800/AI/NIAID NIH HHS/United States
- AI35707/AI/NIAID NIH HHS/United States
- AI AI035800/AI/NIAID NIH HHS/United States
- R01 AI035800/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases