Sending signals dynamically - PubMed (original) (raw)
Review
Sending signals dynamically
Robert G Smock et al. Science. 2009.
Abstract
Proteins mediate transmission of signals along intercellular and intracellular pathways and between the exterior and the interior of a cell. The dynamic properties of signaling proteins are crucial to their functions. We discuss emerging paradigms for the role of protein dynamics in signaling. A central tenet is that proteins fluctuate among many states on evolutionarily selected energy landscapes. Upstream signals remodel this landscape, causing signaling proteins to transmit information to downstream partners. New methods provide insight into the dynamic properties of signaling proteins at the atomic scale. The next stages in the signaling hierarchy-how multiple signals are integrated and how cellular signaling pathways are organized in space and time-present exciting challenges for the future, requiring bold multidisciplinary approaches.
Figures
Fig. 1
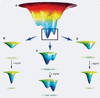
Energy landscapes can be remodeled in several ways to alter protein dynamics and enable them to communicate signaling information. (A) Schematic illustration of the energy landscape available to a protein from higher energy (red) to lower energy (blue). Folding to the native state occurs as the large ensemble of non-native states moves down the energy funnel to the native state. The boxed region encloses conformational states that are energetically accessible and will be sampled under physiological conditions, given thermal fluctuations. (B) One way that a signal can remodel the energy landscape is to narrow the size of the ensemble of states in a single energy well. This reduces the dynamics of the protein, leading to a structural rigidification of the same average conformation. (C) Alternatively, a protein may exist in equilibrium between two distinct conformational states, and an incoming signal can alter the relative energies of the two states, leading to a redistribution of their occupancies. (D) A slight variation on (C) may occur if the sampling of a higher-energy state in the absence of ligand provides a partial pathway toward a signal-induced conformation, as shown by partially overlapping wells of the two states. In the landscape shown, the higher-energy state is narrowed and shifted somewhat in structure upon interaction with a signal.
Fig. 2
Potential pathways of signal transmission within isolated PDZ domains. Similar networks linking the peptide-binding site (bound peptide shown in green sticks) to distal surfaces have been identified in PDZ domains by a number of approaches. (A) Patterns of evolutionarily coupled residues conserved among PDZ family members (shown in red spacefill) connect the peptide-binding site to a distal surface of the domain (12). (B) A network of residues identified by analysis of thermal fluctuations in PDZ-domain crystal structures is shown in blue spacefill, with the bound peptide in green sticks (15). (C) A pathway comprising residues in a PDZ domain, showing dipeptide-induced changes in their side-chain dynamics, is shown in yellow spacefill, with the peptide ligand in green sticks. (13) (D) A dynamic network identified by a molecular dynamics simulation restrained by experimental parameters (14) is shown in spacefill. Unexpectedly, one portion of the network shows enhanced flexibility upon peptide binding (orange) and, conversely, another region (red near the peptide binding pocket) shows reduced dynamics. (E) Domain-domain interaction between two PDZ domains appears to make use of the same basic pathways identified within an isolated PDZ domain. Residues shown as blue spheres were directly implicated in interdomain interactions in a phosphotyrosine phosphatase BL PDZ domain, and a set distal to these showed increased dynamics (red spheres) (42). (F) The interdomain interface identified in a crystal structure (PDB code 1NF3) of a complex of the regulatory Cdc42 (blue cartoon) and the Par-6 PDZ domain (white cartoon) also coincides with the surface of the PDZ domain that is connected to the peptide binding site by many of the identified intradomain networks.
Fig. 3
Examples of how proteins take advantage of intrinsic dynamic properties to respond to an incoming signal. (A) Multiple signals can be integrated to create a response. Here, in the case of calmodulin, first calcium (yellow circles) is sensed by binding to the EF hand subdomains, which favors binding to a target molecule such as myosin light chain kinase (red circle). Binding of the target to one site on calmodulin leads to enhanced likelihood of binding to a second binding site (24). (B) Intramolecular autoinhibition can occur such that a downstream target interaction site is occluded. Opening of the autoinhibitory domain (here shown for Vav) can be favored by an upstream signal such as phosphorylation (29). (C) An intrinsically disordered region (IDR) can confer binding diversity on a signaling protein, enabling two targets to be recognized (35). (D) IDRs also enable a “fly-casting” mechanism of binding by allowing stepwise association with a target (37). (E) In some cases, multivalent binding can be mediated by an IDR that harbors several potential binding sites (small red circles) that each transiently occupy a single site on the target (39). (F) Dynamic domain-domain rearrangements can be triggered by signals such as phosphorylation (45). (G) Disruption of complexes can reveal signals for any of several downstream outcomes, such as relocalization in the cell and degradation (47). (H) Receptor complexes enable the possibility of higher-order modulation of responses to two different signaling ligands (shown as red circle and yellow square) (50).
Similar articles
- Simultaneous prediction of binding free energy and specificity for PDZ domain-peptide interactions.
Crivelli JJ, Lemmon G, Kaufmann KW, Meiler J. Crivelli JJ, et al. J Comput Aided Mol Des. 2013 Dec;27(12):1051-65. doi: 10.1007/s10822-013-9696-9. Epub 2013 Dec 5. J Comput Aided Mol Des. 2013. PMID: 24305904 Free PMC article. - Hippo pathway-independent restriction of TAZ and YAP by angiomotin.
Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Chan SW, et al. J Biol Chem. 2011 Mar 4;286(9):7018-26. doi: 10.1074/jbc.C110.212621. Epub 2011 Jan 11. J Biol Chem. 2011. PMID: 21224387 Free PMC article. - Use of restrained molecular dynamics to predict the conformations of phosphorylated receiver domains in two-component signaling systems.
Foster CA, West AH. Foster CA, et al. Proteins. 2017 Jan;85(1):155-176. doi: 10.1002/prot.25207. Epub 2016 Nov 20. Proteins. 2017. PMID: 27802580 Free PMC article. - Intrinsic disorder in cell signaling and gene transcription.
Tantos A, Han KH, Tompa P. Tantos A, et al. Mol Cell Endocrinol. 2012 Jan 30;348(2):457-65. doi: 10.1016/j.mce.2011.07.015. Epub 2011 Jul 18. Mol Cell Endocrinol. 2012. PMID: 21782886 Review. - PDZ domains: folding and binding.
Jemth P, Gianni S. Jemth P, et al. Biochemistry. 2007 Jul 31;46(30):8701-8. doi: 10.1021/bi7008618. Epub 2007 Jul 10. Biochemistry. 2007. PMID: 17620015 Review.
Cited by
- Molecular Dynamics Simulations Reveal the Mechanisms of Allosteric Activation of Hsp90 by Designed Ligands.
Vettoretti G, Moroni E, Sattin S, Tao J, Agard DA, Bernardi A, Colombo G. Vettoretti G, et al. Sci Rep. 2016 Apr 1;6:23830. doi: 10.1038/srep23830. Sci Rep. 2016. PMID: 27032695 Free PMC article. - Molecular assemblies of the catalytic domain of SOS with KRas and oncogenic mutants.
Moghadamchargari Z, Shirzadeh M, Liu C, Schrecke S, Packianathan C, Russell DH, Zhao M, Laganowsky A. Moghadamchargari Z, et al. Proc Natl Acad Sci U S A. 2021 Mar 23;118(12):e2022403118. doi: 10.1073/pnas.2022403118. Proc Natl Acad Sci U S A. 2021. PMID: 33723061 Free PMC article. - Discovery of intramolecular signal transduction network based on a new protein dynamics model of energy dissipation.
Ma CW, Xiu ZL, Zeng AP. Ma CW, et al. PLoS One. 2012;7(2):e31529. doi: 10.1371/journal.pone.0031529. Epub 2012 Feb 20. PLoS One. 2012. PMID: 22363664 Free PMC article. - Protein dynamics at Eph receptor-ligand interfaces as revealed by crystallography, NMR and MD simulations.
Qin H, Lim L, Song J. Qin H, et al. BMC Biophys. 2012 Jan 25;5:2. doi: 10.1186/2046-1682-5-2. BMC Biophys. 2012. PMID: 22277260 Free PMC article. - Investigating the Role of Large-Scale Domain Dynamics in Protein-Protein Interactions.
Delaforge E, Milles S, Huang JR, Bouvier D, Jensen MR, Sattler M, Hart DJ, Blackledge M. Delaforge E, et al. Front Mol Biosci. 2016 Sep 13;3:54. doi: 10.3389/fmolb.2016.00054. eCollection 2016. Front Mol Biosci. 2016. PMID: 27679800 Free PMC article. Review.
References
- Yu H, et al. Science. 2008;322:104. published online 21 August 2008 (10.1126/science.1158684) - PubMed
- Perutz MF, Mathews FS. J. Mol. Biol. 1966;21:199. - PubMed
- Henzler-Wildman K, Kern D. Nature. 2007;450:964. - PubMed
- Linderstrom-Lang K, Schellman J. In: The Enzymes. Boyer P, editor. vol. 1. New York: Academic Press; 1959. pp. 443–510.
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008515/GM/NIGMS NIH HHS/United States
- OD000945/OD/NIH HHS/United States
- DP1 OD000945-03/OD/NIH HHS/United States
- GM027616/GM/NIGMS NIH HHS/United States
- R01 GM027616-30/GM/NIGMS NIH HHS/United States
- DP1 OD000945/OD/NIH HHS/United States
- R01 GM027616/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources