Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model - PubMed (original) (raw)
Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model
Heng Du et al. Neurobiol Aging. 2011 Mar.
Abstract
Mitochondrial stress is one of the early features of Alzheimer disease (AD). Mitochondrial Aβ has been linked to mitochondrial toxicity. Our recent study demonstrated that cyclophilin D (CypD) mediated mitochondrial permeability transition pore (mPTP) is an important mechanism for neuronal and synaptic stress induced by both Aβ and oxidative stress. In transgenic AD-type mice overexpressing mutant amyloid precursor protein (APP) and Aβ (mAPP), CypD deficiency improves mitochondrial and synaptic function and learning/memory up to 12 months old. Here we provide evidence of the protective effects of CypD deficiency in aged AD mice (22-24 months). Cyp D deficient mAPP mice demonstrate less calcium-induced mitochondrial swelling, increased mitochondrial calcium uptake capacity, preserved mitochondrial respiratory function and improved spatial learning/memory even in old age (known to be the age for late stage AD pathology and synaptic dysfunction). These data demonstrate that abrogation of CypD results in persistent life-long protection against Aβ toxicity in an Alzheimer's disease mouse model, thereby suggesting that blockade of CypD may be of benefit for Alzheimer disease treatment.
Copyright © 2009 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statement: We have no conflicts of interest to disclose. We have no contract relating this research with any organization that could benefit financially from our research.
Figures
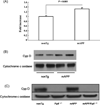
Fig. 1
CypD expression in aged transgenic mice. Panel (A) densitometry of immunoreactive bands using NIH imageJ program (n = 8 mice per group; P < 0.001). Panels (B and C). Representative images of Western blots for CypD. Western blot for cytochrome c oxidase was used as a protein loading control showing an equal amount of protein added to each lane.
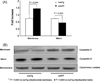
Fig. 2
Cerebral Aβ levels in transgenic mice at age of 22–24 months. Panel (A) densitometry of Aβ immunoreactive bands by using NIH imageJ program. Panel (B) Representative images of Western blot for Aβ and β-actin. β-Actin was used as a protein loading control (n = 4–5 per group).
Fig. 3
Effect of CypD deficiency on mitochondrial swelling and calcium uptake capacity. (A) Comparison of mitochondrial swelling among Tg mice cortical mitochondria (n = 4–5 mice per group; *P < 0.05 vs. nonTg mitochondria; #P < 0.001 vs. mAPP or nonTg mitochondria panel A1). Mitochondrial swelling was induced by the addition of 500 nmol calcium per mg mitochondrial protein. The addition of CSA completely rescued mAPP mitochondria from calcium-induced swelling (*P < 0.001 vs. mAPP/Ppif−/− mitochondria or mAPP mitochondria incubated with CSA panel A2). The calcium buffering capacity of mAPP mitochondria is much lower than that of nonTg, mAPP/Ppif−/− mitochondria or mAPP mitochondria with CSA (*P < 0.01; n = 4–6 mice per group panel B).
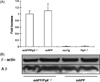
Fig. 4
CypD translocation. Mitochondrial membrane and matrix were isolated from cortices of the indicated Tg mice and then subjected to Western blotting for CypD (n = 4 mice per group). Panel (A) indicates densitometry of CypD immunoreactive bands and panel (B) shows representative images of Western blotting for CypD and cytochrome c oxidase. Cytochrome c oxidase was used as a protein loading control indicating an equal amount of mitochondrial protein employed for each of the experiments.
Fig. 5
Effect of CypD deficiency on mitochondrial respiratory function and cytochrome c oxidase activity in mAPP mice. Respiration control rate (RCR) in the cortical mitochondria from the indicated Tg mice at 22–24 months old (panel (A1)). RCR values are shown in panel (A2) (n = 4–5 mice per group, *P < 0.005 vs. nonTg, mAPP/Ppif−/− or Ppif−/− mitochondria and #P < 0.01 vs. nonTg or Ppif−/− mitochondria). Cytochrome c oxidase activity in the indicated Tg mice at 22–24 months old (panel (B1)). Value for cytochrome c oxidase activity (panel (B2)) (n = 4–5 mice per group, *P < 0.05 vs. other groups of mice).
Fig. 6
Effect of CypD deficiency on spatial learning and memory. Mice were tested in a 6-arm radialwater maze.mAPPmice made more errors in locating the hidden platform than other groups of mice (*P < 0.01). mAPP/Ppif−/− mice demonstrated better performance than mAPP mice but worse than nonTg and Ppif−/− mice (#P < 0.01; n = 6–7 mice per group).
Similar articles
- Synergistic exacerbation of mitochondrial and synaptic dysfunction and resultant learning and memory deficit in a mouse model of diabetic Alzheimer's disease.
Wang Y, Wu L, Li J, Fang D, Zhong C, Chen JX, Yan SS. Wang Y, et al. J Alzheimers Dis. 2015;43(2):451-63. doi: 10.3233/JAD-140972. J Alzheimers Dis. 2015. PMID: 25096625 Free PMC article. - Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease.
Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Du H, et al. Nat Med. 2008 Oct;14(10):1097-105. doi: 10.1038/nm.1868. Epub 2008 Sep 21. Nat Med. 2008. PMID: 18806802 Free PMC article. - Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer's neurons.
Guo L, Du H, Yan S, Wu X, McKhann GM, Chen JX, Yan SS. Guo L, et al. PLoS One. 2013;8(1):e54914. doi: 10.1371/journal.pone.0054914. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23382999 Free PMC article. - Mitochondrial permeability transition pore in Alzheimer's disease: cyclophilin D and amyloid beta.
Du H, Yan SS. Du H, et al. Biochim Biophys Acta. 2010 Jan;1802(1):198-204. doi: 10.1016/j.bbadis.2009.07.005. Epub 2009 Jul 16. Biochim Biophys Acta. 2010. PMID: 19616093 Free PMC article. Review. - Mitochondrial permeability transition pore is a potential drug target for neurodegeneration.
Rao VK, Carlson EA, Yan SS. Rao VK, et al. Biochim Biophys Acta. 2014 Aug;1842(8):1267-72. doi: 10.1016/j.bbadis.2013.09.003. Epub 2013 Sep 18. Biochim Biophys Acta. 2014. PMID: 24055979 Free PMC article. Review.
Cited by
- Mitochondrial permeability transition pore contributes to mitochondrial dysfunction in fibroblasts of patients with sporadic Alzheimer's disease.
Pérez MJ, Ponce DP, Aranguiz A, Behrens MI, Quintanilla RA. Pérez MJ, et al. Redox Biol. 2018 Oct;19:290-300. doi: 10.1016/j.redox.2018.09.001. Epub 2018 Sep 4. Redox Biol. 2018. PMID: 30199818 Free PMC article. - Interaction of Tau, IL-6 and mitochondria on synapse and cognition following sevoflurane anesthesia in young mice.
Zhang J, Dong Y, Lining Huang, Xu X, Liang F, Soriano SG, Zhang Y, Xie Z. Zhang J, et al. Brain Behav Immun Health. 2020 Aug 28;8:100133. doi: 10.1016/j.bbih.2020.100133. eCollection 2020 Oct. Brain Behav Immun Health. 2020. PMID: 34589883 Free PMC article. - Identification of a Small Molecule Cyclophilin D Inhibitor for Rescuing Aβ-Mediated Mitochondrial Dysfunction.
Valasani KR, Sun Q, Fang D, Zhang Z, Yu Q, Guo Y, Li J, Roy A, ShiDu Yan S. Valasani KR, et al. ACS Med Chem Lett. 2016 Jan 6;7(3):294-9. doi: 10.1021/acsmedchemlett.5b00451. eCollection 2016 Mar 10. ACS Med Chem Lett. 2016. PMID: 26985318 Free PMC article. - Glucocorticoid-driven mitochondrial damage stimulates Tau pathology.
Du F, Yu Q, Swerdlow RH, Waites CL. Du F, et al. Brain. 2023 Oct 3;146(10):4378-4394. doi: 10.1093/brain/awad127. Brain. 2023. PMID: 37070763 Free PMC article. - PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer's disease.
Du F, Yu Q, Yan S, Hu G, Lue LF, Walker DG, Wu L, Yan SF, Tieu K, Yan SS. Du F, et al. Brain. 2017 Dec 1;140(12):3233-3251. doi: 10.1093/brain/awx258. Brain. 2017. PMID: 29077793 Free PMC article.
References
- Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim. Biophys. Acta. 2004;1742(1–3):81–87. - PubMed
- Aleardi AM, Benard G, Augereau O, Malgat M, Talbot JC, Mazat JP, Letellier T, Dachary-Prigent J, Solaini GC, Rossignol R. Gradual alteration of mitochondrial structure and function by beta-amyloids: importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release. J. Bioenerg. Biomembr. 2005;37(4):207–225. - PubMed
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, Luddy JS, Lue LF, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, Stern DM, Du Yan SS. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23(20):4096–4105. - PMC - PubMed
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–662. - PubMed
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 2005;280(19):18558–18561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG017490/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- P050 AG08702/AG/NIA NIH HHS/United States
- P01AG17490/AG/NIA NIH HHS/United States
- P50 AG008702-17/AG/NIA NIH HHS/United States
- P01 AG017490-11/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases