Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration - PubMed (original) (raw)
Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration
Marie Potier et al. FASEB J. 2009 Aug.
Abstract
The identity of store-operated calcium (Ca(2+)) entry (SOCE) channels in vascular smooth muscle cells (VSMCs) remains a highly contentious issue. Whereas previous studies have suggested that SOCE in VSMCs is mediated by the nonselective transient receptor potential canonical (TRPC) 1 protein, the identification of STIM1 and Orai1 as essential components of I(CRAC), a highly Ca(2+)-selective SOCE current in leukocytes, has challenged that view. Here we show that cultured proliferative migratory VSMCs isolated from rat aorta (called "synthetic") display SOCE with classic features, namely inhibition by 2-aminoethoxydiphenyl borate, ML-9, and low concentrations of lanthanides. On store depletion, synthetic VSMCs and A7r5 cells display currents with characteristics of I(CRAC). Protein knockdown of either STIM1 or Orai1 in synthetic VSMCs greatly reduced SOCE, whereas Orai2, Orai3, TRPC1, TRPC4, and TRPC6 knockdown had no effect. Orai1 knockdown reduced I(CRAC) in synthetic VSMCs and A7r5 cells. Synthetic VSMCs showed up-regulated STIM1/Orai1 proteins and SOCE compared with quiescent freshly isolated VSMC. Knockdown of STIM1 and Orai1 inhibited synthetic VSMC proliferation and migration, whereas STIM2, Orai2, and Orai3 knockdown had no effect. To our knowledge, these results are the first to show I(CRAC) in VSMCs and resolve a long-standing controversy by identifying CRAC as the elusive VSMC SOCE channel important for proliferation and migration.
Figures
Figure 1.
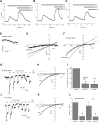
Characterization of SOCE and _I_CRAC in synthetic VSMCs. A) Thapsigargin (TG) (2 μM) activates SOCE in synthetic VSMCs that is sensitive to 5 μM Gd3+ (_n_=72). B, C) Similar inhibition of SOCE was achieved with 50 μM 2-APB (B) (_n_=49) and 50 μM ML-9 (C) (_n_=26). D) Store depletion by dialysis of 20 mM BAPTA activates a relatively small current in 10 mM Ca2+. E) I/V relation of current shown in D before and after subtraction of the sweep right after break-in. F) Representative data of I/V relations of DVF current before and after subtraction of Gd3+-insensitive current. G) Store depletion by dialysis of 20 mM BAPTA in synthetic VSMC activates _I_CRAC-like currents, revealed in DVF solutions that were inhibited by 5 μM Gd3+; sweeps represented are indicated by differently shaded asterisks. H) I/V relations in Ca2+-containing solution (10 mM), before and after Gd3+ addition. I) Statistical analysis of monovalent _I_CRAC inhibition by 5 μM Gd3+, normalized to control. J, K) Similar results to those in G, H were obtained with the A7r5 smooth muscle cell line. L) Statistical analysis of monovalent _I_CRAC inhibition by 5 μM Gd3+, shown as current densities. All Ca2+ imaging traces are average data from several cells analyzed simultaneously. Data are representative of 3 to 4 independent experiments.
Figure 2.
SOCE and _I_CRAC in synthetic VSMCs are inhibited by 5 μM 2-APB. A, B) Average data in HEK293 cells (A) (_n_=51) and RBL cells (B) (_n_=53). 2-APB at 5 μM potentiates SOCE activated by submaximal concentrations of thapsigargin (TG) (100 nM), whereas 50 μM 2-APB inhibits SOCE in these cells. C, D) Thapsigargin (2 μM) activates SOCE in synthetic VSMCs (C) (_n_=12) and the A7r5 cell line (D) (_n_=14), which is inhibited by 5 μM 2-APB. E, F) Dose response of 2-APB inhibition in synthetic VSMCs (representing average from 12–54 cells). G, H) Inhibition of _I_CRAC recorded in DVF solutions by 5 μM 2-APB. Ca2+ imaging data are representative of 4 independent coverslips, with each coverslip analyzing 21–30 cells. Patch-clamp data are representative of 4 independent experiments.
Figure 3.
Successful mRNA and protein knockdown of STIM1, Orai, and TRPC using siRNA. A–F) Western blot analysis probing for decrease protein levels of STIM1 (A), Orai (B, C), and TRPC isoforms (D–F) after RNA silencing. Actin was used as a control, and densitometry was performed using ImageJ software (for Orai3 blots, densitometry is shown for siOrai3-1). Extended versions of Western blots are shown in Supplemental Fig. 2. G) qPCR assessing effect of specific siRNA on Orai2 mRNA levels. Western blotting data are representative of 3 independent experiments; qPCR data are representative of 6 independent experiments. Wt, wild type.
Figure 4.
STIM1 and Orai1 mediate SOCE in synthetic VSMCs. Average SOCE in response to 2 μM thapsigargin (TG) after siRNA knockdown of STIM1 from one coverslip (A) and statistical analysis of several cells from different coverslips are shown (B) (control, _n_=25; siSTIM1, _n_=19 cells). Similar data are shown for Orai1 (C, D) (control, _n_=22; siOrai1, _n_=16 cells), Orai2 (E, F) (control, _n_=24; siOrai2, _n_=23 cells), and Orai3 (G, H) (control, _n_=34; siOrai3, _n_=20 cells). No siRNA had any significant effect on Ca2+ release. Data are representative of at least 4 independent experiments.
Figure 5.
TRPC1, TRPC4, and TRPC6 are not involved in synthetic VSMC SOCE. Average SOCE in response to 2 μM thapsigargin (TG) after siRNA knockdown of TRPC1 from one coverslip (A), and statistical analysis of several cells from different coverslips are shown (B) (control, _n_=70; siTRPC1, _n_=145 cells). Similar data are shown for TRPC4 (C, D) (control, _n_=51; siTRPC4, _n_=42 cells), TRPC6 (E, F) (control, _n_=33; siTRPC6, _n_=27 cells), and simultaneous knockdown of TRPC1/4/6 (G, H) (control, _n_=21; siTRPC1/4/6, _n_=24). No siRNA had any significant effect on Ca2+ release. Data are representative of at least 4 independent experiments (9 independent experiments for siTRPC1).
Figure 6.
Orai1 knockdown inhibits _I_CRAC in synthetic VSMCs and A7r5 cells. A–D) Silencing of Orai1 inhibits _I_CRAC recorded in DVF solutions in synthetic VSMCs (A, C). Similar results were obtained in A7r5 cells (B, D). STIM1 knockdown in A7r5 cells also inhibits _I_CRAC recorded in DVF solutions (B, D). E, F) Statistical analysis in synthetic VSMCs (E) and A7r5 cells (F), based on 3 to 4 independent experiments. G) Effect of 5 μM 2-APB on SOCE in wild-type synthetic VSMCs transfected with GFP (_n_=30) and in same cells transfected with CFP-Orai1 alone (_n_=16) or CFP-Orai1 + eYFP-STIM1 (_n_=28). Data are representative of 4 independent experiments. TG, thapsigargin.
Figure 7.
SOCE and STIM1/Orai1 protein levels are increased in proliferative migratory VSMCs. A, B) Thapsigargin (TG) (2 μM) activates a much bigger SOCE in synthetic VSMCs (Synth.VSMC; _n_=154) than in quiescent freshly dispersed VSMCs (Quiesc.VSMC; _n_=98). Traces are averages from 25 to 30 cells and are representative of 6 independent experiments, each analyzing 21–35 cells. C) Synthetic VSMCs express higher levels of STIM1 protein compared with quiescent freshly isolated VSMCs. D) Similar results were seen in regard to Orai1 expression. Western blotting data are representative of 4 independent experiments. RBL mast cells were used as controls for STIM1 protein expression.
Figure 8.
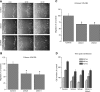
STIM1 and Orai1 knockdown inhibits synthetic VSMC proliferation and migration. A) Bright-field views of scratch wound migration assay at different times postwound in synthetic VSMCs transfected with either crambled control siRNA or siRNA against either STIM1 or Orai1. B, C) Data from 3 independent experiments (4 wells/condition), quantified and analyzed as described in Materials and Methods, presented as percentage of control. D) Proliferation in VSMCs transfected with scrambled control siRNA, siSTIM1, siOrai1, and siSTIM1 + siOrai1, assayed as described in Materials and Methods. Data are presented as percentage of control and are representative of 5 independent experiments in 96-well plates (12 independent wells/transfection).
Similar articles
- Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration.
Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd'heuil D, Trebak M. Bisaillon JM, et al. Am J Physiol Cell Physiol. 2010 May;298(5):C993-1005. doi: 10.1152/ajpcell.00325.2009. Epub 2010 Jan 27. Am J Physiol Cell Physiol. 2010. PMID: 20107038 Free PMC article. - Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation.
Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Abdullaev IF, et al. Circ Res. 2008 Nov 21;103(11):1289-99. doi: 10.1161/01.RES.0000338496.95579.56. Epub 2008 Oct 9. Circ Res. 2008. PMID: 18845811 Free PMC article. - Airway smooth muscle STIM1 and Orai1 are upregulated in asthmatic mice and mediate PDGF-activated SOCE, CRAC currents, proliferation, and migration.
Spinelli AM, González-Cobos JC, Zhang X, Motiani RK, Rowan S, Zhang W, Garrett J, Vincent PA, Matrougui K, Singer HA, Trebak M. Spinelli AM, et al. Pflugers Arch. 2012 Nov;464(5):481-92. doi: 10.1007/s00424-012-1160-5. Epub 2012 Sep 27. Pflugers Arch. 2012. PMID: 23014880 Free PMC article. - Store-Independent Orai Channels Regulated by STIM.
Zhang X, Gueguinou M, Trebak M. Zhang X, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review.
Cited by
- STIM1 controls endothelial barrier function independently of Orai1 and Ca2+ entry.
Shinde AV, Motiani RK, Zhang X, Abdullaev IF, Adam AP, González-Cobos JC, Zhang W, Matrougui K, Vincent PA, Trebak M. Shinde AV, et al. Sci Signal. 2013 Mar 19;6(267):ra18. doi: 10.1126/scisignal.2003425. Sci Signal. 2013. PMID: 23512989 Free PMC article. - STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion.
Motiani RK, Hyzinski-García MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, Matrougui K, Mongin AA, Trebak M. Motiani RK, et al. Pflugers Arch. 2013 Sep;465(9):1249-60. doi: 10.1007/s00424-013-1254-8. Epub 2013 Mar 21. Pflugers Arch. 2013. PMID: 23515871 Free PMC article. - Transient receptor potential channels in the vasculature.
Earley S, Brayden JE. Earley S, et al. Physiol Rev. 2015 Apr;95(2):645-90. doi: 10.1152/physrev.00026.2014. Physiol Rev. 2015. PMID: 25834234 Free PMC article. Review. - Attenuated mesangial cell proliferation related to store-operated Ca2+ entry in aged rat: the role of STIM 1 and Orai 1.
Shen B, Zhu J, Zhang J, Jiang F, Wang Z, Zhang Y, Li J, Huang D, Ke D, Ma R, Du J. Shen B, et al. Age (Dordr). 2013 Dec;35(6):2193-202. doi: 10.1007/s11357-013-9511-5. Epub 2013 Jan 20. Age (Dordr). 2013. PMID: 23334602 Free PMC article. - Essential Role of Smooth Muscle STIM1 in Hypertension and Cardiovascular Dysfunction.
Kassan M, Ait-Aissa K, Radwan E, Mali V, Haddox S, Gabani M, Zhang W, Belmadani S, Irani K, Trebak M, Matrougui K. Kassan M, et al. Arterioscler Thromb Vasc Biol. 2016 Sep;36(9):1900-9. doi: 10.1161/ATVBAHA.116.307869. Epub 2016 Jul 28. Arterioscler Thromb Vasc Biol. 2016. PMID: 27470514 Free PMC article.
References
- Putney J W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. - PubMed
- Putney J W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. - PubMed
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. - PubMed
- Parekh A B, Putney J W., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K22ES014729/ES/NIEHS NIH HHS/United States
- R01 HL092510/HL/NHLBI NIH HHS/United States
- R01 HL049426/HL/NHLBI NIH HHS/United States
- K22 ES014729-03S1/ES/NIEHS NIH HHS/United States
- K22 ES014729/ES/NIEHS NIH HHS/United States
- R01 HL097111-01A1/HL/NHLBI NIH HHS/United States
- K22 ES014729-03S2/ES/NIEHS NIH HHS/United States
- K22 ES014729-03/ES/NIEHS NIH HHS/United States
- R01 HL097111/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous