Glycogen synthase kinase 3: more than a namesake - PubMed (original) (raw)
Review
Glycogen synthase kinase 3: more than a namesake
Geetha Vani Rayasam et al. Br J Pharmacol. 2009 Mar.
Abstract
Glycogen synthase kinase 3 (GSK3), a constitutively acting multi-functional serine threonine kinase is involved in diverse physiological pathways ranging from metabolism, cell cycle, gene expression, development and oncogenesis to neuroprotection. These diverse multiple functions attributed to GSK3 can be explained by variety of substrates like glycogen synthase, tau protein and beta catenin that are phosphorylated leading to their inactivation. GSK3 has been implicated in various diseases such as diabetes, inflammation, cancer, Alzheimer's and bipolar disorder. GSK3 negatively regulates insulin-mediated glycogen synthesis and glucose homeostasis, and increased expression and activity of GSK3 has been reported in type II diabetics and obese animal models. Consequently, inhibitors of GSK3 have been demonstrated to have anti-diabetic effects in vitro and in animal models. However, inhibition of GSK3 poses a challenge as achieving selectivity of an over achieving kinase involved in various pathways with multiple substrates may lead to side effects and toxicity. The primary concern is developing inhibitors of GSK3 that are anti-diabetic but do not lead to up-regulation of oncogenes. The focus of this review is the recent advances and the challenges surrounding GSK3 as an anti-diabetic therapeutic target.
Figures
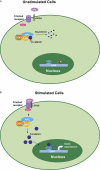
Figure 2
Schematic illustration of role of GSK3 in Wnt signalling. In the unstimulated cells β catenin is present in a multi-protein complex with GSK3, Axin and APC. Phosphorylation of β catenin by GSK3 targets β catenin to proteosome-mediated degradation. In stimulated cells binding of Wnt to its receptor Frizzled and its co-receptor LRP5/6 leads to activation of Dishevelled (Dvl) leading to inhibition of phosphorylation of β catenin by the multimeric complex and its stabilization and accumulation in the cytosol. Stabilized β catenin translocates to the nucleus and in association with transcription factors LEF/TCF leads to increase in gene expression of target genes such as c-myc. APC, adenomatous polyposis coli; GSK3, glycogen synthase kinase 3; LEF/TCF, lymphoid enhancer factor/T cell factor; LRP, LDL receptor related protein.
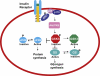
Figure 1
Schematic representation of insulin-signalling events and role of GSK3. Binding of insulin to its receptor leads to autophosphorylation, followed by binding of IRS that is phosphorylated leading to activation of PI3 kinase and Akt/PKB that phosphorylates GSK3. Inactive phosphorylated GSK3 relieves glycogen synthase inhibition and eIF2B phosphorylation, leading to active form of GS and eIF2B, which leads to increase in glycogen and protein synthesis. eIF2B, eukaryotic initiation factor 2B; GS, glycogen synthase; GSK3, glycogen synthase kinase 3; IRS, insulin receptor substrates; PKB, protein kinase B.
Similar articles
- Discovery and development of GSK3 inhibitors for the treatment of type 2 diabetes.
Wagman AS, Johnson KW, Bussiere DE. Wagman AS, et al. Curr Pharm Des. 2004;10(10):1105-37. doi: 10.2174/1381612043452668. Curr Pharm Des. 2004. PMID: 15078145 Review. - Acute selective glycogen synthase kinase-3 inhibition enhances insulin signaling in prediabetic insulin-resistant rat skeletal muscle.
Dokken BB, Sloniger JA, Henriksen EJ. Dokken BB, et al. Am J Physiol Endocrinol Metab. 2005 Jun;288(6):E1188-94. doi: 10.1152/ajpendo.00547.2004. Epub 2005 Jan 25. Am J Physiol Endocrinol Metab. 2005. PMID: 15671078 - The role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes.
Kaidanovich O, Eldar-Finkelman H. Kaidanovich O, et al. Expert Opin Ther Targets. 2002 Oct;6(5):555-61. doi: 10.1517/14728222.6.5.555. Expert Opin Ther Targets. 2002. PMID: 12387679 Review. - Use of lithium and SB-415286 to explore the role of glycogen synthase kinase-3 in the regulation of glucose transport and glycogen synthase.
MacAulay K, Hajduch E, Blair AS, Coghlan MP, Smith SA, Hundal HS. MacAulay K, et al. Eur J Biochem. 2003 Sep;270(18):3829-38. doi: 10.1046/j.1432-1033.2003.03777.x. Eur J Biochem. 2003. PMID: 12950267 - GSK3: a key target for the development of novel treatments for type 2 diabetes mellitus and Alzheimer disease.
Gao C, Hölscher C, Liu Y, Li L. Gao C, et al. Rev Neurosci. 2011 Dec 21;23(1):1-11. doi: 10.1515/rns.2011.061. Rev Neurosci. 2011. PMID: 22718609 Review.
Cited by
- Glycogen synthase kinase 3 regulates expression of nuclear factor-erythroid-2 related transcription factor-1 (Nrf1) and inhibits pro-survival function of Nrf1.
Biswas M, Kwong EK, Park E, Nagra P, Chan JY. Biswas M, et al. Exp Cell Res. 2013 Aug 1;319(13):1922-1931. doi: 10.1016/j.yexcr.2013.04.013. Epub 2013 Apr 23. Exp Cell Res. 2013. PMID: 23623971 Free PMC article. - Differential effects of glycogen synthase kinase 3 (GSK3) inhibition by lithium or selective inhibitors in the central nervous system.
Caberlotto L, Carboni L, Zanderigo F, Andreetta F, Andreoli M, Gentile G, Razzoli M. Caberlotto L, et al. Naunyn Schmiedebergs Arch Pharmacol. 2013 Oct;386(10):893-903. doi: 10.1007/s00210-013-0893-9. Epub 2013 Jun 22. Naunyn Schmiedebergs Arch Pharmacol. 2013. PMID: 23793101 - GSK-3β inhibition protects mesothelial cells during experimental peritoneal dialysis through upregulation of the heat shock response.
Rusai K, Herzog R, Kuster L, Kratochwill K, Aufricht C. Rusai K, et al. Cell Stress Chaperones. 2013 Sep;18(5):569-79. doi: 10.1007/s12192-013-0410-6. Epub 2013 Mar 14. Cell Stress Chaperones. 2013. PMID: 23494401 Free PMC article. - GSK3β-mediated Keap1-independent regulation of Nrf2 antioxidant response: A molecular rheostat of acute kidney injury to chronic kidney disease transition.
Lu M, Wang P, Qiao Y, Jiang C, Ge Y, Flickinger B, Malhotra DK, Dworkin LD, Liu Z, Gong R. Lu M, et al. Redox Biol. 2019 Sep;26:101275. doi: 10.1016/j.redox.2019.101275. Epub 2019 Jul 17. Redox Biol. 2019. PMID: 31349118 Free PMC article. - Inhibition of glycogen synthase kinase 3beta (GSK3beta) decreases inflammatory responses in brain endothelial cells.
Ramirez SH, Fan S, Zhang M, Papugani A, Reichenbach N, Dykstra H, Mercer AJ, Tuma RF, Persidsky Y. Ramirez SH, et al. Am J Pathol. 2010 Feb;176(2):881-92. doi: 10.2353/ajpath.2010.090671. Epub 2010 Jan 7. Am J Pathol. 2010. PMID: 20056834 Free PMC article.
References
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. - PubMed
- van Amerongen R, Berns A. Re-evaluating the role of Frat in Wnt-signal transduction. Cell Cycle. 2005;4:1065–1072. - PubMed
- van Amerongen R, van der Gulden H, Bleeker F, Jonkers J, Berns A. Characterization and functional analysis of the murine Frat2 gene. J Biol Chem. 2004;279:26967–26974. - PubMed
- Asuni AA, Hooper C, Reynolds CH, Lovestone S, Anderton BH, Killick R. GSK3alpha exhibits beta-catenin and tau directed kinase activities that are modulated by Wnt. Eur J Neurosci. 2006;24:3387–3392. - PubMed
- Baggio LL, Drucker DJ. Therapeutic approaches to preserve islet mass in type 2 diabetes. Annu Rev Med. 2006;57:265–281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases