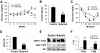Butyrate improves insulin sensitivity and increases energy expenditure in mice - PubMed (original) (raw)
. 2009 Jul;58(7):1509-17.
doi: 10.2337/db08-1637. Epub 2009 Apr 14.
Affiliations
- PMID: 19366864
- PMCID: PMC2699871
- DOI: 10.2337/db08-1637
Butyrate improves insulin sensitivity and increases energy expenditure in mice
Zhanguo Gao et al. Diabetes. 2009 Jul.
Abstract
Objective: We examined the role of butyric acid, a short-chain fatty acid formed by fermentation in the large intestine, in the regulation of insulin sensitivity in mice fed a high-fat diet.
Research design and methods: In dietary-obese C57BL/6J mice, sodium butyrate was administrated through diet supplementation at 5% wt/wt in the high-fat diet. Insulin sensitivity was examined with insulin tolerance testing and homeostasis model assessment for insulin resistance. Energy metabolism was monitored in a metabolic chamber. Mitochondrial function was investigated in brown adipocytes and skeletal muscle in the mice.
Results: On the high-fat diet, supplementation of butyrate prevented development of insulin resistance and obesity in C57BL/6 mice. Fasting blood glucose, fasting insulin, and insulin tolerance were all preserved in the treated mice. Body fat content was maintained at 10% without a reduction in food intake. Adaptive thermogenesis and fatty acid oxidation were enhanced. An increase in mitochondrial function and biogenesis was observed in skeletal muscle and brown fat. The type I fiber was enriched in skeletal muscle. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression was elevated at mRNA and protein levels. AMP kinase and p38 activities were elevated. In the obese mice, supplementation of butyrate led to an increase in insulin sensitivity and a reduction in adiposity.
Conclusions: Dietary supplementation of butyrate can prevent and treat diet-induced insulin resistance in mouse. The mechanism of butyrate action is related to promotion of energy expenditure and induction of mitochondria function.
Figures
FIG. 1.
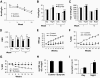
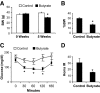
Energy metabolism in response to sodium butyrate. Butyrate increased energy expenditure in C57BL/B6 mice. Energy expenditure was examined using the metabolic chamber at the 1st week and the 10th week on high-fat diet (16 weeks in age). In this study, sodium butyrate was used at 5% wt/wt in high-fat diet. A: Food intake. Food intake was monitored daily for 5 days at each time point. Average daily food intake (g) was converted into kilocalories and normalized with body weight (kg) and 24 h. B: Energy expenditure measured as kilocalories per kilogram lean mass per hour. C: Oxygen consumption measured as milliliters volume oxygen per kilogram lean mass per hour. D: Substrate utilization. This is expressed by respiratory exchange ratio (RER), which is the volume ratio of oxygen consumed versus CO2 exhaled. E: Body weight (BW). F: Body fat content in percentage of body weight. This was determined by nuclear magnetic resonance. G: Body muscle content in percentage of body weight. H: Lipid in feces. Feces were collected in the cages during a 24-h period on high-fat diet at 12 weeks. Total lipids were extracted and quantified (P > 0.05, n = 5). I: Spontaneous physical activity. The frequency of horizontal movement (X) was shown for day and night at 10 weeks on a high-fat diet. For A_–_D, and I, n = 8 in the control or butyrate group. For E_–_G, n = 10 in the control or butyrate group. Values are the means ± SE. *P < 0.05, **P < 0.001 by Student's t test. □, Control; ■, butyrate.
FIG. 2.
Insulin sensitivity in butyrate-treated mice. A: Fasting glucose. Tail vein blood was used for glucose assay after 16 h fasting during the period of high-fat diet feeding. B: Fasting insulin. The insulin level was determined at 16 weeks on high-fat diet in fasting condition with a Lincoplex kit (MADPK model). C: Intraperitoneal insulin tolerance in butyrate-treated mice. Intraperitoneal insulin tolerance testing was performed at 12 weeks on high-fat diet (at 16 weeks of age). In A_–_C, data are the means ± SE (n = 9). *P < 0.05, **P < 0.001 by Student's t test. D: HOMA-IR. After an overnight fast, blood glucose and insulin were measured and used to determine insulin sensitivity through HOMA-IR (IR = fasting insulin mU/ml × fasting glucose mg/dl ÷ 405). Values are the means ± SE (n = 8 mice). **P < 0.001. E: Insulin signaling. The gastrocnemius muscle was isolated after insulin (0.75 units/kg) injection in mice for 30 min and used to prepare the whole-cell lysate for immunoblot. The mice on high-fat diet for 13 weeks were used in the signaling assay. F: Signal quantification. The blot signal in E was quantified and presented after normalization with protein loading. **P < 0.001 (n = 2). IRS, insulin receptor substrate.
FIG. 3.
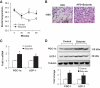
Brown adipose tissue response to sodium butyrate. A: Adaptive thermogenesis in cold environment. Rectum temperature was measured when the mice were exposed to 4°C ambient temperature in a cold room at 10 weeks on high-fat diet. Details of the procedure are described in
research design and methods
. B: Hematoxylin and eosin staining in BAT. The staining was conducted in BAT collected at 13 weeks on high-fat diet. Photograph was taken at ×100 magnification. C: mRNA expression in BAT. BAT was collected at 13 weeks on high-fat diet. Gene expression was examined by qRT-PCR. mRNA of PGC-1α and UCP-1 in brown fat of mice treated with butyrate was measured. D: Immunoblot of protein in BAT. BAT was collected at 13 weeks of butyrate treatment. The whole-cell lysate (100 μg) was resolved in SDS-PAGE and blotted with PGC-1α and UCP-1 antibodies. Data are the means ± SE (n = 9 mice). *P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 4.
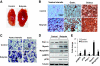
Oxidative fiber in skeletal muscle. A: Vastus lateralis muscle. The tissue was isolated from mice that were fed high-fat diet for 13 weeks. B: Oxidative fiber (type I fibers) in serial cryostat sections of muscle. The muscle tissue slides were made from vastus lateralis, gastrocnemius (gastr.), and soleus muscle. They were stained with antibody against type I myosin heavy chain for oxidative fibers, as indicated by the brown color. The photograph was taken at ×20 magnification. C: Succinate dehydrogenase staining of oxidative fibers. The oxidative fibers were stained in serial cryostat sections of the vastus lateralis and gastrocnemius (gastr.) muscle as indicated by dark blue color in the photomicrograph. D and E: Quantification of proteins in immunoblot. The whole-cell lysate was prepared from muscle tissues and analyzed in an immunoblot. Signals for PGC-1α, type I myosin heavy chain, myoglobin, phosphorylated AMPK (pAMPK), and phosphorylated p38 (pP38) were blotted with specific antibodies. A representative blot is shown. Relative signal strength was quantified for each band and expressed in the bar figure. Results are the means ± SE (n = 8 mice). *P < 0.01, **P < 0.001 (vs. control). (A high-quality digital representation of this figure is available in the online issue.)
FIG. 5.
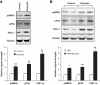
Effect of butyrate on L6 muscle cells and liver tissues. A: AMPK and PGC-1α in L6 cells. Differentiated L6 myotubes were starved in 0.25% BSA and Dulbecco's modified Eagle's medium overnight. The cells were treated with 500 μmol/l of sodium butyrate for 4 h and analyzed in an immunoblot. A mean value of triplicate experiments is shown in the bar figure. B: AMPK and PGC-1α in liver. The whole-cell lysate was prepared from liver tissues collected from mice on high-fat diet (HFD) for 13 weeks and analyzed in an immunoblot. In the experiments, phosphorylated AMP kinase (pAMPK), phosphorylated p38 (pP38), and PGC-1α were blotted with the specific antibodies. A representative blot is shown. A mean value of five mice is shown in the bar figure (n = 5). *P < 0.05; **P < 0.001.
FIG. 6.
Mitochondrial function and blood lipids. Vastus lateralis muscle and blood samples were collected from mice at 13 weeks on high-fat diet (18 weeks in age) and examined for fatty acid oxidation, gene expression, and blood lipids. A: Fatty acid oxidation in muscle. The _y_-axis represents fold change in 14C-labled CO2. B: Gene expression in muscle. Relative fold change in mRNA was used to indicate gene expression. C: Mitochondrial DNA COX-I (cytochrome c oxidase I) determined by SYBR Green RT-PCR. D: Fatty acid oxidation in L6 cells. Fully differentiated L6 cells were treated with 500 μmol/l butyrate for 16 h, and fatty acid oxidation was measured. E: Gene expression in L6 cells. Relative fold change in mRNA was used to indicate gene expression. F: Butyrate in serum. G: Histone deacetylase activity in muscle. H: Triglyceride in blood. I: Total cholesterol in blood. Data are the means ± SE (n = 6). *P < 0.05; **P < 0.001.
FIG. 7.
Treatment of obesity with butyrate. Obesity was induced in C57BL/6J mice fed a high-fat diet for 16 weeks (21 weeks in age). The obese mice were then treated with butyrate through food supplementation for 5 weeks. A: Body weight (BW). Body weight was shown at the beginning and end of the 5-week butyrate treatment. B: Fat content. Fat content was determined in the body using nuclear magnetic resonance at the end of the 5-week treatment with butyrate. C: Intraperitoneal insulin tolerance. At the end of 5 weeks, intraperitoneal insulin tolerance testing was performed after a 4-h fast. D: HOMA-IR. Values are the means ± SE (n = 8 in each group). *P < 0.05.
References
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J: Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006; 127: 1109– 1122 - PubMed
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA: Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006; 444: 337– 342 - PMC - PubMed
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J: Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006; 439: 484– 489 - PubMed
- Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, Porco JA, Jr, Lowell BB: Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab 2006; 3: 417– 427 - PubMed
- Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ: The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 2002; 217: 133– 139 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1 P30 DK072476/DK/NIDDK NIH HHS/United States
- DK68036/DK/NIDDK NIH HHS/United States
- R56 DK068036/DK/NIDDK NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- R01 DK068036/DK/NIDDK NIH HHS/United States
- P50 AT02776-020002/AT/NCCIH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases