Notch signaling promotes airway mucous metaplasia and inhibits alveolar development - PubMed (original) (raw)
Notch signaling promotes airway mucous metaplasia and inhibits alveolar development
J Sawalla Guseh et al. Development. 2009 May.
Abstract
The airways are conduits that transport atmospheric oxygen to the distal alveolus. Normally, airway mucous cells are rare. However, diseases of the airway are often characterized by mucous metaplasia, in which there are dramatic increases in mucous cell numbers. As the Notch pathway is known to regulate cell fate in many contexts, we misexpressed the active intracellular domain of the mouse Notch1 receptor in lung epithelium. Notch misexpression resulted in an increase in mucous cells and a decrease in ciliated cells in the airway. Similarly, mouse embryonic tracheal explants and adult human airway epithelium treated with Notch agonists displayed increased mucous cell numbers and decreased ciliated cell numbers. Notch antagonists had the opposite effect. Notably, Notch antagonists blocked IL13-induced mucous metaplasia. IL13 has a well-established role as an inflammatory mediator of mucous metaplasia and functions through Stat6-mediated gene transcription. We found that Notch ligands, however, are able to cause mucous metaplasia in Stat6-null cultured trachea, thus identifying a novel pathway that stimulates mucous metaplasia. Notch signaling may therefore play an important role in airway disease and, by extension, Notch antagonists may have therapeutic value. Conversely, in the distal lung, Notch misexpression prevented the differentiation of alveolar cell types. Instead, the distal lung formed cysts composed of cells that were devoid of alveolar markers but that expressed some, but not all, markers of proximal airway epithelium. Occasional distal cystic cells appeared to differentiate into normal proximal airway cells, suggesting that ectopic Notch signaling arrests the normal differentiation of distal lung progenitors before they initiate an alveolar program.
Figures
Fig. 1.
Constitutive Notch expression in embryonic lung results in distal cyst formation. (A) Strategy to express activated Notch intracellular domain (NotchIC) in developing lung epithelium. The triangles represent_loxP_ sites. (B,B′) Lungs from E18.5 NotchIC transgenic pups and control littermates (B). GFP transgene activation is evident in NotchIC transgenic lungs and absent in control littermates (B′). (C,C′) H&E staining of E18.5 control littermate (C) and NotchIC transgenic (C′) lungs reveals dilated cysts in place of alveolar saccules. Scale bars: 100 μm in C,C′.
Fig. 2.
Constitutive Notch expression inhibits differentiation of distal alveolar saccules. (A,A′) E18.5 Hes1 immunohistochemistry in control (A) and NotchIC transgenic (A′) lungs. The brackets indicate control saccules (A) and corresponding cysts in transgenic animals (A′). BADJ, bronchioalveolar duct junction. (B,B′) E18.5 Nkx2.1 immunohistochemistry (red) in control (B) and NotchIC transgenic (B′) lungs. (C,C′) E18.5 surfactant protein C (SPC) immunohistochemistry (red) in control (C) and transgenic NotchIC lungs (C′). GFP-positive (green) cells do not express SPC (arrow). However, SPC-positive cells persist when GFP is absent (arrowheads). The bracket indicates a GFP-negative saccule. (D,D′) E18.5 E-cadherin immunohistochemistry (red) in control (D) and NotchIC-gfp transgenic lungs (D′). E-cadherin expression stops at the BADJ in control lungs but persists throughout the cystic epithelium in transgenic lungs. (E,E′) E18.5 smooth muscle-myosin (SMM) immunohistochemistry (red) in control (E) and NotchIC-gfp (green) transgenic lungs (E′). SMM expression is restricted to proximal airways (dashed line) in control lung but is ectopically expressed surrounding distal cysts in transgenic lungs. (F,F′) E18.5 CC10 immunohistochemistry (red) demonstrates normal CC10 patterning in transgenic animals compared with controls (inset) (F). A, airway. (F′) Scattered CC10-positive cells (arrowheads) are found in GFP+ transgenic cysts. (G,G′) E18.5 BrdU immunohistochemistry (red) of control (G) and NotchIC-gfp (green) transgenic (G′) lungs after a 2 hour BrdU pulse. BrdU incorporation (percentage shown) is reduced in cystic epithelial cells compared with control alveolar epithelial cells. (H,H′) TUNEL staining (red, percentage shown) of E18.5 control (H) and NotchIC transgenic (H′) lungs reveals an increase in apoptosis in transgenic lungs compared with control. Scale bars: 100 μm.
Fig. 3.
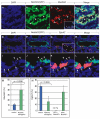
Constitutive Notch activation leads to more mucous cells and fewer ciliated cells in E18.5 airway epithelium. (A-D) E18.5 immunohistochemistry of NotchIC airways. Transgene expression (green, B) is correlated with increased numbers of Muc5AC-positive cells (red, C). (C) Inset demonstrates the rare presence of mucous cells in control airway epithelium. (D) Color merge reveals that mucous cells co-label with GFP (yellow). Arrowheads indicate mucin-positive cells. (E-H) E18.5 immunohistochemistry of transgenic Notch airway epithelium shows few ciliated cells. Notch-transgene-expressing cells that are GFP-positive (green, F) lack EphA7 (red, G). (G) The inset demonstrates normal ciliated cell numbers in control epithelium as marked by EphA7 (arrowheads point to ciliated cells in red). (H) Rare residual ciliated cells marked by EphA7 (red) lack GFP (green) transgene expression. (I-L) The yellow-boxed regions from E-H are shown at high magnification in I-L, respectively. Merge reveals that GFP-positive NotchIC transgenic cells (arrowheads, green) are distinct from EphA7-positive (arrows, red) cells. (M,N) Quantification of Muc5AC+ mucus cells and EphA7+ ciliated cells in E18.5 airway epithelium demonstrates that Notch activation is associated with increased mucous cell differentiation (M) (_P_=0.006) but fewer ciliated cells (N) (_P_=0.003). GFP-negative cells in transgenic lungs showed normal ciliated cell differentiation, whereas GFP+ cells showed virtually no ciliated cell differentiation. Scale bars: 100 μm.
Fig. 4.
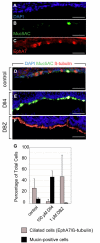
Notch agonists and antagonists alter ciliated and mucous cell numbers in mouse tracheal explants. (A-C) E14.5 mouse tracheas (A) cultured in vitro for 10 days display normal mucous (B) and ciliated cell (C) differentiation. (D-F) Immunohistochemistry of tracheal explants: mucous cells (green) and ciliated cells (red) were present in control explants (D). Culture with the Notch ligand Dll4 results in increased mucous cell differentiation (green, E). Addition of a Notch signaling antagonist, DBZ, results in increased ciliated cell differentiation (red, F). (G) Incubation of tracheal explants in Dll4 increases mucous cells while decreasing ciliated cells. Addition of DBZ, the Notch antagonist, results in the near absence of Muc5AC-positive cells. Scale bars: 25 μm.
Fig. 5.
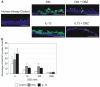
Notch antagonists decrease mucous cell differentiation in human airway epithelial cultures. (A) Human airway cultures incubated with Dll4 or IL13 (middle column) show substantial Muc5AC (green) immunostaining compared with control (left panel). DBZ addition to Dll4 or IL13 (right-hand column) decreased Muc5AC staining. (B) Increasing concentrations of DBZ decreased Muc5AC staining in control cultures (white) and in those cultures incubated with either Dll4 (gray) or IL13 (black). Scale bars: 100 μm.
Fig. 6.
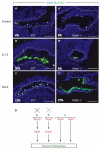
Stat6 is not necessary for DLL4-induced mucous metaplasia. (A-C) Immunohistochemistry of control tracheas that were harvested at E14.5 and cultured for 7 days. Tracheas displayed normal, low levels of mucous differentiation, identified by Muc5AC staining (green) (A). Addition of IL13 (B) or DLL4 (C) induced mucous metaplasia. (A′-C′) Immunohistochemistry of _Stat6_–/– tracheas that were harvested at E14.5 and cultured for 7 days. Tracheas displayed normal, low levels of mucous differentiation (A′). Addition of IL13 (B′) failed to induce mucous metaplasia as predicted. Addition of DLL4 (C′) induced mucous metaplasia. (D) Possible models for the interaction of Notch and the IL13/Stat6 signaling pathway, which both result in mucous metaplasia. Notch signaling cannot be upstream of Stat6 activation as Notch-induced metaplasia occurs in _Stat6_-null trachea. Notch signaling might be downstream of Stat6 signaling, although this is unlikely because Stat6 activation is not associated with Notch target induction. Therefore, Notch and Stat6 signaling may represent two parallel pathways for inducing mucous metaplasia. Scale bars: 100 μm.
Fig. 7.
Models of Notch action in mouse lung development. (A) Notch misexpression increases mucous cell differentiation and inhibits ciliated cell differentiation in proximal airway epithelium. (B) Notch downregulation is required for alveolar development. Constitutive Notch misexpression inhibits alveolar development and results in a dilated cystic epithelium. (C) Schematic representations of possible common and dual lineage progenitor models for lung development. In the common lineage model, a single progenitor lineage produces proximal and distal cell types. Notch signaling could block the transition from a proximal progenitor to a distal progenitor (a) or could block the differentiation of an already established distal progenitor (b) in this model. In the dual lineage model, in which there are distinct proximal and distal progenitors, Notch signaling may specifically block the differentiation of distal progenitors, whereas it would only modulate the specific cell-fate distribution of proximal progenitor progeny.
Similar articles
- IL-17A induces signal transducers and activators of transcription-6-independent airway mucous cell metaplasia.
Newcomb DC, Boswell MG, Sherrill TP, Polosukhin VV, Boyd KL, Goleniewska K, Brody SL, Kolls JK, Adler KB, Peebles RS Jr. Newcomb DC, et al. Am J Respir Cell Mol Biol. 2013 Jun;48(6):711-6. doi: 10.1165/rcmb.2013-0017OC. Am J Respir Cell Mol Biol. 2013. PMID: 23392574 Free PMC article. - Notch signaling prevents mucous metaplasia in mouse conducting airways during postnatal development.
Tsao PN, Wei SC, Wu MF, Huang MT, Lin HY, Lee MC, Lin KM, Wang IJ, Kaartinen V, Yang LT, Cardoso WV. Tsao PN, et al. Development. 2011 Aug;138(16):3533-43. doi: 10.1242/dev.063727. Development. 2011. PMID: 21791528 Free PMC article. - Involvement of the p38 MAPK pathway in IL-13-induced mucous cell metaplasia in mouse tracheal epithelial cells.
Fujisawa T, Ide K, Holtzman MJ, Suda T, Suzuki K, Kuroishi S, Chida K, Nakamura H. Fujisawa T, et al. Respirology. 2008 Mar;13(2):191-202. doi: 10.1111/j.1440-1843.2008.01237.x. Respirology. 2008. PMID: 18339016 - Notch Signaling: Linking Embryonic Lung Development and Asthmatic Airway Remodeling.
Hussain M, Xu C, Ahmad M, Yang Y, Lu M, Wu X, Tang L, Wu X. Hussain M, et al. Mol Pharmacol. 2017 Dec;92(6):676-693. doi: 10.1124/mol.117.110254. Epub 2017 Oct 12. Mol Pharmacol. 2017. PMID: 29025966 Review.
Cited by
- Stem cells and cell therapies in lung biology and diseases: conference report.
Weiss DJ, Bates JH, Gilbert T, Liles WC, Lutzko C, Rajagopal J, Prockop D. Weiss DJ, et al. Ann Am Thorac Soc. 2013 Oct;10(5):S25-44. doi: 10.1513/AnnalsATS.201304-089AW. Ann Am Thorac Soc. 2013. PMID: 23869447 Free PMC article. No abstract available. - Lung organogenesis.
Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Warburton D, et al. Curr Top Dev Biol. 2010;90:73-158. doi: 10.1016/S0070-2153(10)90003-3. Curr Top Dev Biol. 2010. PMID: 20691848 Free PMC article. Review. - Progenitor potential of lung epithelial organoid cells in a transplantation model.
Louie SM, Moye AL, Wong IG, Lu E, Shehaj A, Garcia-de-Alba C, Ararat E, Raby BA, Lu B, Paschini M, Bronson RT, Kim CF. Louie SM, et al. Cell Rep. 2022 Apr 12;39(2):110662. doi: 10.1016/j.celrep.2022.110662. Cell Rep. 2022. PMID: 35417699 Free PMC article. - SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production.
Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. Chen G, et al. J Clin Invest. 2009 Oct;119(10):2914-24. doi: 10.1172/JCI39731. Epub 2009 Sep 14. J Clin Invest. 2009. PMID: 19759516 Free PMC article. - Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury.
Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Volckaert T, et al. J Clin Invest. 2011 Nov;121(11):4409-19. doi: 10.1172/JCI58097. Epub 2011 Oct 10. J Clin Invest. 2011. PMID: 21985786 Free PMC article.
References
- Borges, M., Linnoila, R. I., van de Velde, H. J., Chen, H., Nelkin, B. D., Mabry, M., Baylin, S. B. and Ball, D. W. (1997). An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 386, 852-855. - PubMed
- Cardoso, W. V. (2001). Molecular regulation of lung development. Annu. Rev. Physiol. 63, 471-494. - PubMed
- Conlon, R. A., Reaume, A. G. and Rossant, J. (1995). Notch1 is required for the coordinate segmentation of somites. Development 121, 1533-1545. - PubMed
- Dang, T. P., Eichenberger, S., Gonzalez, A., Olson, S. and Carbone, D. P. (2003). Constitutive activation of Notch3 inhibits terminal epithelial differentiation in lungs of transgenic mice. Oncogene 22, 1988-1997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous