Transcriptional regulation of monocyte chemotactic protein-1 release by endothelin-1 in human airway smooth muscle cells involves NF-kappaB and AP-1 - PubMed (original) (raw)
Transcriptional regulation of monocyte chemotactic protein-1 release by endothelin-1 in human airway smooth muscle cells involves NF-kappaB and AP-1
Amy M Sutcliffe et al. Br J Pharmacol. 2009 Jun.
Abstract
Background and purpose: Endothelin-1 (ET-1) is implicated in airway inflammation in asthma, but the mechanisms of its effects are poorly understood. We studied the effect of ET-1 on expression of the chemokine, monocyte chemotactic protein-1 (MCP-1), in primary cultures of human airway smooth muscle cells.
Experimental approach: MCP-1 release was measured by elisa. Pharmacological antagonists/inhibitors, reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blotting were used to study ET receptors and kinase cascades. Transcriptional regulation was studied by real-time RT-PCR, transient transfection studies and chromatin immunoprecipitation assay. Major findings were confirmed in cells from three donors and mechanistic studies in cells from one donor.
Key results: ET-1 increased MCP-1 release through an ET(A) and ET(B) receptor-dependent mechanism. ET-1 increased MCP-1 mRNA levels but not mRNA stability suggesting it was acting transcriptionally. ET-1 increased the activity of an MCP-1 promoter-reporter construct. Serial deletions of the MCP-1 promoter mapped ET-1 effects to a region between -213 and -128 base pairs upstream of the translation start codon, containing consensus sequences for activator protein-1 (AP-1) and nuclear factor-kappaB (NF-kappaB). ET-1 promoted binding of AP-1 c-Jun subunit and NF-kappaB p65 subunit to the MCP-1 promoter. Blocking the inhibitor of kappaB kinase-2 with 2-[(aminocarbonyl)amino]-5-[4-fluorophenyl]-3-thiophenecarboxamide (TPCA-1) decreased ET-1-stimulated MCP-1 production. p38 and p44/p42 mitogen-activated protein kinases were involved in upstream signalling.
Conclusions and implications: ET-1 regulated MCP-1 transcriptionally, via NF-kappaB and AP-1. The upstream signalling involved ET(A), ET(B) receptors, p38 and p44/p42 mitogen-activated protein kinases. These may be targets for novel asthma therapies.
Figures
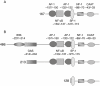
Figure 1
The human MCP-1 gene is regulated by a distal enhancer region containing two NF-κB consensus sequences (not shown), and a more complex proximal promoter region from −486 to −32. Numbers refer to the 5′ nucleotide relative to the translation start site. (A) MCP-1 promoter. The MCP-1 promoter construct used in this study consisted of the 167 bp upstream of the translational start codon driving a luciferase reporter gene. (B) MCP-1 promoter deletion series. The MCP-1 promoter deletion series consisted of the 486, 213 and 128 bp upstream of the translational start codon driving a luciferase reporter gene. AP-1, activator protein-1; GAS, gamma-activated site; IRIS, interferon response inhibitory sequence; MCP-1, monocyte chemotactic protein-1; NF-1, nuclear factor-1; NF-κB: nuclear factor-κB; Sp1, small protein-1.
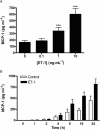
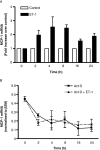
Figure 2
(A) Concentration–response of ET-1-stimulated MCP-1 production by HASMC. Cells were treated for 24 h with ET-1 at the concentrations indicated. ET-1 significantly increased MCP-1 release (P < 0.001). ***P < 0.001 compared with unstimulated cells. Each bar represents group mean (SE) derived from 13 replicates in four independent experiments (_n_= 3 different primary donors). (B) Time–course of ET-1-stimulated MCP-1 production by HASMC. Cells were treated with ET-1 (10 ng·mL−1) for the indicated times. ET-1 significantly increased MCP-1 release from HASMC in a time-dependent manner (ET-1 vs. control: P < 0.001. Interaction between ET-1 and time: P < 0.001). Each bar represents group mean (SE) derived from 18 replicates in seven independent experiments (_n_= 3 different primary donors). ET-1, endothelin-1; HASMC, human airway smooth muscle cells; MCP-1, monocyte chemotactic protein-1.
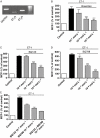
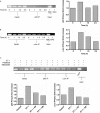
Figure 3
(A) ETA and ETB receptor mRNA is expressed in HASMC. Representative gel from one of three independent experiments (_n_= 1). (B) The dual selective ET receptor antagonist bosentan concentration-dependently inhibited ET-1-stimulated MCP-1 production (P < 0.001). ***P < 0.001 compared with ET-1-stimulated cells. Each bar represents group mean (SE) derived from 11 replicates in three independent experiments (_n_= 1). (C) The selective ETA receptor antagonist BQ123 concentration-dependently inhibited ET-1-stimulated MCP-1 production (P < 0.001). ***P < 0.001 compared with ET-1-stimulated cells. (D) The selective ETB receptor antagonist BQ788 concentration-dependently inhibited ET-1-stimulated MCP-1 production (P < 0.001). *_P_= 0.03; ***P < 0.001 compared with ET-1-stimulated cells. (E) BQ123, BQ788 and both inhibitors in combination (10−7 mol·L−1) significantly inhibited ET-1-stimulated MCP-1 production (P < 0.001). For interaction _P_= 0.053. (C–E) each bar represents group mean (SE) derived from six replicates in two independent experiments (_n_= 1). ET-1, endothelin-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HASMC, human airway smooth muscle cells; MCP-1, monocyte chemotactic protein-1.
Figure 4
(A) Effect of PD98059 (MEK inhibitor) on ET-1-stimulated MCP-1 production. PD98059 (20 µmol·L−1) inhibited ET-1-stimulated MCP-1 production. ***P < 0.001 compared with cells treated with ET-1 alone. Each bar represents group mean (SE) derived from 11 replicates in three independent experiments (_n_= 1). (B) Western blotting showing time-dependent phosphorylation of p44/p42 MAPK by ET-1. An increase in phosphorylation of p44/p42 MAPK is seen at 5 min and is sustained until 30 min. Representative blot from one of four independent experiments (_n_= 1). (C) Effect of SB203580 (p38 MAPK inhibitor) on ET-1-stimulated MCP-1 production. SB203580 (20 µmol·L−1) inhibited ET-1-stimulated MCP-1 production. ***P < 0.001 compared with cells treated with ET-1 alone. Each bar represents group mean (SE) derived from 16 replicates in two independent experiments (_n_= 1). (D) Western blotting showing time-dependent phosphorylation of p38 MAPK by ET-1. An increase in phosphorylation of p38 MAPK is seen at 5 min and is sustained until 30 min. Representative blot from one of three independent experiments (_n_= 1). (E) The JNK inhibitor SP600125 (10 µmol·L−1) had no effect on ET-1-stimulated MCP-1 production. Each bar represents group mean (SE) derived from 10 replicates in three independent experiments (_n_= 1). (F) The PI3 kinase inhibitors wortmannin (10−7 mol·L−1) and LY294002 (10−6 mol·L−1) had no effect on ET-1-stimulated MCP-1 production. Each bar represents group mean (SE) derived from eight replicates in two independent experiments (_n_= 1). (A, C, E and F) MCP-1 production is shown as a percentage of MCP-1 production by control (untreated) cells. ET-1, endothelin-1; JNK, c-Jun NH2-terminal kinase; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemotactic protein-1; MEK, mitogen-activated protein kinase kinase; PI3 kinase, phosphatidyl inositol 3-kinase.
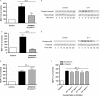
Figure 5
(A) ET-1 increased expression of MCP-1 mRNA measured by qRT-PCR (P < 0.001 compared with control cells). The effect was time-dependent with the maximal effect of ET-1 observed at around 4 h stimulation (for interaction between ET-1 and time, _P_= 0.003). MCP-1 mRNA was normalized to the housekeeping gene β2M. The results are expressed as fold increase over unstimulated controls at the same time point. Each bar represents group mean (SE) derived from 12 replicates in four independent experiments (_n_= 1). (B) Effect of ET-1 on MCP-1 mRNA stability. ET-1 treatment had no effect on the rate of decay of MCP-1 transcripts, after treatment with actinomycin D (Act D). MCP-1 mRNA was normalized to the housekeeping gene β2M. Each point represents group mean (SE) derived from six replicates in three independent experiments (_n_= 1). β2M, β2 microglobulin; ET-1, endothelin-1; MCP-1, monocyte chemotactic protein-1; qRT-PCR, quantitative real-time reverse transcriptase-polymerase chain reaction.
Figure 6
(A) Effect of ET-1 on MCP-1 promoter activity. ET-1 stimulated MCP-1 promoter-driven luciferase activity (_P_= 0.03 compared with controls). Each bar represents group mean (SE) derived from 18 replicates in three independent experiments (_n_= 1). (B) Effect of ET-1 on MCP-1 enhancer activity. Although there was a trend towards an effect of ET-1 on MCP-1 enhancer-driven luciferase activity, this was not statistically significant (_P_= 0.106 compared with controls). Each bar represents group mean (SE) derived from 16–26 replicates in three to six independent experiments (_n_= 1). (C) HASMC were transiently transfected with serially deleted MCP-1 promoter constructs consisting of the 486, 213 or 128 bp upstream of the translational start codon and stimulated with ET-1 for 6 h. ET-1 stimulated luciferase activity driven by the 486 and 213 constructs but had no effect on the 128 construct. **_P_= 0.008; ***P < 0.001. Each bar represents group mean (SE) derived from 18 replicates in three independent experiments (_n_= 1). (D and E) Effect of ET-1 on NF-κB (D) and AP-1 (E) reporter-driven luciferase activity following 6 h treatment with ET-1. *_P_= 0.03 compared with control. Each bar represents group mean (SE) derived from 18 replicates in three independent experiments (D) or 12 replicates in two independent experiments (E, _n_= 1). (F) Effect of the JNK inhibitor SP600125 on ET-1-stimulated MCP-1 promoter activity. HASMC transiently transfected with the wild-type MCP-1 promoter construct were stimulated with ET-1 for 6 h in the absence or presence of SP600125. SP600125 had no effect on ET-1-stimulated MCP-1 promoter activity. **_P_= 0.009 compared with controls. Each bar represents group mean (SE) derived from 12 replicates in two independent experiments (_n_= 1). (A–F) expressed as fold increase in luciferase activity in ET-1-treated cells compared with control (untreated) cells. (G) Cells were pre-incubated with the IKK-2 inhibitor TPCA-1 for 30 min prior to 24 h stimulation with ET-1. TPCA-1 concentration-dependently inhibited ET-1-stimulated MCP-1 production (four-parameter logistic regression). Each point represents group mean (SE) derived from 12 replicates in four independent experiments (_n_= 1). AP-1, activator protein-1; ET-1, endothelin-1; HASMC, human airway smooth muscle cells; IKK-2, inhibitor of κB kinase-2; JNK, c-Jun NH2-terminal kinase; MCP-1, monocyte chemotactic protein-1; NF-κB, nuclear factor-κB; n.s., not significant; TPCA-1, 2-[(aminocarbonyl)amino]-5-[4-fluorophenyl]-3-thiophenecarboxamide.
Figure 7
ChIP assays showing effect of ET-1 on in vivo binding of NF-κB p65 subunit (A) and AP-1 c-Jun subunit (B) to the MCP-1 promoter. ET-1 promoted in vivo binding of p65 and c-Jun to the MCP-1 promoter. (C) ET-1-stimulated binding of p65 and c-Jun to the MCP-1 promoter at 1 h was inhibited by PD98059 (20 µmol·L−1) and SB203580 (20 µmol·L−1). (A–C) Representative gels, with accompanying densitometry, from one of two independent experiments (_n_= 1). The density of the immunoprecipitated band (IP) was normalized to that of the input control at the same time point, and expressed as fold increase over time zero (time–course) or unstimulated cells (inhibitor studies). AP-1, activator protein-1; ChIP, chromatin immunoprecipitation; ET-1, endothelin-1; MCP-1, monocyte chemotactic protein-1; NAC, no antibody control; NF-κB, nuclear factor-κB.
Similar articles
- High glucose conditions induce upregulation of fractalkine and monocyte chemotactic protein-1 in human smooth muscle cells.
Dragomir E, Manduteanu I, Calin M, Gan AM, Stan D, Koenen RR, Weber C, Simionescu M. Dragomir E, et al. Thromb Haemost. 2008 Dec;100(6):1155-65. Thromb Haemost. 2008. PMID: 19132243 - Involvement of p38 MAPK, JNK, p42/p44 ERK and NF-kappaB in IL-1beta-induced chemokine release in human airway smooth muscle cells.
Wuyts WA, Vanaudenaerde BM, Dupont LJ, Demedts MG, Verleden GM. Wuyts WA, et al. Respir Med. 2003 Jul;97(7):811-7. doi: 10.1016/s0954-6111(03)00036-2. Respir Med. 2003. PMID: 12854631 - Cardioprotective signaling by endothelin.
Schorlemmer A, Matter ML, Shohet RV. Schorlemmer A, et al. Trends Cardiovasc Med. 2008 Oct;18(7):233-9. doi: 10.1016/j.tcm.2008.11.005. Trends Cardiovasc Med. 2008. PMID: 19232951 Free PMC article. Review. - New asthma drugs acting on gene expression.
Popescu FD. Popescu FD. J Cell Mol Med. 2003 Oct-Dec;7(4):475-86. doi: 10.1111/j.1582-4934.2003.tb00251.x. J Cell Mol Med. 2003. PMID: 14754517 Free PMC article. Review.
Cited by
- All-trans retinoic acid reverses epithelial-mesenchymal transition in paclitaxel-resistant cells by inhibiting nuclear factor kappa B and upregulating gap junctions.
Shi G, Zheng X, Wu X, Wang S, Wang Y, Xing F. Shi G, et al. Cancer Sci. 2019 Jan;110(1):379-388. doi: 10.1111/cas.13855. Epub 2018 Nov 27. Cancer Sci. 2019. PMID: 30375704 Free PMC article. - Monocyte-Induced Prostate Cancer Cell Invasion is Mediated by Chemokine ligand 2 and Nuclear Factor-κB Activity.
Lindholm PF, Sivapurapu N, Jovanovic B, Kajdacsy-Balla A. Lindholm PF, et al. J Clin Cell Immunol. 2015 Apr;6(2):308. doi: 10.4172/2155-9899.1000308. J Clin Cell Immunol. 2015. PMID: 26317041 Free PMC article. - NF-κB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions.
Tantiwong P, Shanmugasundaram K, Monroy A, Ghosh S, Li M, DeFronzo RA, Cersosimo E, Sriwijitkamol A, Mohan S, Musi N. Tantiwong P, et al. Am J Physiol Endocrinol Metab. 2010 Nov;299(5):E794-801. doi: 10.1152/ajpendo.00776.2009. Epub 2010 Aug 24. Am J Physiol Endocrinol Metab. 2010. PMID: 20739506 Free PMC article. - NF-κB and AP-1 are required for the lipopolysaccharide-induced expression of MCP-1, CXCL1, and Cx43 in cultured rat dorsal spinal cord astrocytes.
Lu Y, Li B, Xu A, Liang X, Xu T, Jin H, Xie Y, Wang R, Liu X, Gao X, Han Y, Zeng J. Lu Y, et al. Front Mol Neurosci. 2022 Jul 28;15:859558. doi: 10.3389/fnmol.2022.859558. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35966011 Free PMC article. - CCL2 release by airway smooth muscle is increased in asthma and promotes fibrocyte migration.
Singh SR, Sutcliffe A, Kaur D, Gupta S, Desai D, Saunders R, Brightling CE. Singh SR, et al. Allergy. 2014 Sep;69(9):1189-97. doi: 10.1111/all.12444. Epub 2014 Jun 16. Allergy. 2014. PMID: 24931417 Free PMC article.
References
- Adner M, Cardell LO, Sjoberg T, Ottosson A, Edvinsson L. Contractile endothelin-B (ETB) receptors in human small bronchi. Eur Respir J. 1996;9:351–355. - PubMed
- Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50:515–596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous