BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP+-induced toxicity in cultured hippocampal and mesencephalic slices - PubMed (original) (raw)
BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP+-induced toxicity in cultured hippocampal and mesencephalic slices
H Jourdi et al. Neuropharmacology. 2009 Apr.
Abstract
Neurotoxicity is involved in various neurodegenerative diseases including Parkinson's disease (PD), which affects mesencephalic dopaminergic neurons of the substantia nigra (SN). Positive alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor modulators (PARMs, a.k.a. Ampakines, such as CX614) increase brain-derived neurotrophic factor (BDNF) protein levels in vivo and in cultured hippocampal slices. BDNF is a survival factor for various neuronal cell types including mesencephalic dopaminergic neurons. Using cultured mesencephalic and hippocampal slices, we investigated whether preincubation with CX614 could provide neuroprotection against MPP(+) toxicity and whether such neuroprotection was mediated by BDNF. Various treatment protocols were tested to demonstrate CX614-induced neuroprotection against MPP(+). Pretreatment with CX614 significantly reduced MPP(+)-induced toxicity and increased BDNF levels in both hippocampal and mesencephalic cultured slices; CX614 pretreatment for 6 h in hippocampal slices and 24 h in mesencephalic slices was sufficient to produce significant neuroprotection as assessed with lactate dehydrogenase release in slice medium and propidium iodide uptake in slices. Both a BDNF scavenger and an inhibitor of the BDNF receptor TrkB, abrogated CX614-mediated reduction of MPP(+)-induced toxicity. Inhibition of Ca(2+)-activated proteases, calpains, was also protective against MPP(+)-induced toxicity. However, co-application of calpain inhibitor with CX614 abolished CX614-mediated protection, suggesting a dual action of calpains in this model. We conclude that CX614 is neuroprotective against MPP(+)-induced toxicity, an effect mediated by increased BDNF expression and activation of BDNF-dependent signaling pathways. Our results provide support for using PARMs as a new therapy for neurodegenerative disorders, including PD.
Figures
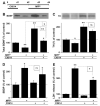
Fig. 1
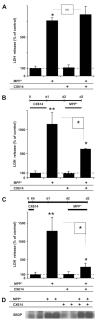
Effects of co-treatment or pre-treatment with CX614 on MPP+-induced LDH release and calpain activation in cultured hippocampal slices. A. Cultured hippocampal slices were treated simultaneously for 24 h with CX614 (50 μM) and MPP+ (250 μM). At the end of treatment, LDH release in the medium was assessed as described in Section2. B. Cultured hippocampal slices were pretreated with CX614 (50 μM) for 24 h (d1), transferred to CX614-free fresh medium for 1 day (d2) before being treated with MPP+ (250 μM) for 1 day (d3). Medium was collected at the end of treatment (d3) and LDH release in the medium was assessed. C. Treatment of cultured hippocampal slices was similar to (B) except that slices were pretreated with CX614 (50 μM) for 6 h only and incubated in CX614-free medium for 42 h before treatment with MPP+. D. Total protein samples from slices treated as in “C” were analyzed by western blotting for accumulation of spectrin breakdown products (SBDP) as a marker of calpain activation. Results are expressed as percent of control and are means ± SEM of 5 experiments with 3–6 samples per condition. Statistical significance was calculated with ANOVA followed by Tukey’s test; *, p < 0.05; **, p < 0.01; ns: not significant.
Fig. 2
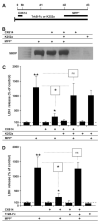
Effects of K252a or TrkB-Fc and CX614 on MPP+-induced calpain activation and LDH release in cultured hippocampal slices. A. All slices were treated according to the protocol shown in the diagram; K252a (0.5 μM) or TrkB-Fc (0.3 μg/ml) treatment was initiated 30 min and 1 h, respectively, before CX614 addition (50 μM; 6 h). Slices were then transferred to CX614-free medium containing K252a or TrkB-Fc. MPP+ (250 μM) was added 42 h later and kept for 1 day (d3). SBDP accumulation was examined by western blotting as an indicator of calpain activation (B). LDH release into the medium (C,D), collected at the end of all treatments, was measured as described in Section2. B. MPP+-induced SBDP accumulation was not reduced by pretreatment with CX614 alone or CX614 + K252a. K252a and CX614 alone did not increase SBDP accumulation. C. K252a treatment eliminated CX614-mediated reduction of MPP+-induced increase in LDH release. D. TrkB-Fc treatment eliminated CX614-mediated reduction of MPP+-induced increase in LDH release. Results are expressed as percent of control and are means ± SEM of 3 experiments with 3–4 samples per condition. Statistical significance was calculated with ANOVA followed by Tukey’s test; *, p < 0.05; **, p < 0.01; ns, not significant.
Fig. 3
Effects of calpain inhibition and CX614 on MPP+ toxicity in cultured hippocampal slices. A. Schematic representation of the protocol used in this experiment; Calpain Inhibitor III (CI3; 10 μM) was added in the culture medium 1 day (d1) before addition of MPP+ (250 μM), which was kept for 2 days (d3). At the end of treatment (d3), medium was collected for LDH analysis (C) and slices were collected for SBDP analysis (B). B. Western blot showing SBDP accumulation from three independent samples from each experimental condition. C. LDH activity in medium collected at the end of treatment (d3). D. CX614 (50 μM) was added at the same time as CI3 and hippocampal slices were treated as in (A) up to day 3 and medium was collected at the end of treatment for LDH analysis as indicated in _Section_2. Results are expressed as percent of control and are means ± SEM of 4 experiments with 3–6 samples per condition. Statistical significance was calculated with ANOVA followed by Tukey’s test; *, p < 0.05; **, p < 0.01; ns, not significant.
Fig. 4
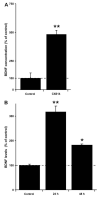
Effects of CX614 treatment on BDNF levels in cultured hippocampal and mesencephalic slices. CX614 (50 μM) treatment of cultured hippocampal (A) and mesencephalic (B) slices was carried out for 6 h and 1 day, respectively. Slices were then transferred to and maintained in fresh medium for 1 day for hippocampal cultures and for 1 and 2 day(s) for mesencephalic cultures. BDNF tissue content was analyzed as indicated in Section 2. Results are expressed as percent of control and are means ± SEM of 6–8 experiments. Statistics calculated by unpaired Student’s _t_-test; *, p < 0.05; **, p < 0.01.
Fig. 5
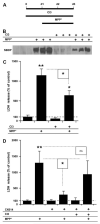
Effects of CX614 on BDNF, TH and SBDP levels and LDH release following MPP+ treatment of cultured mesencephalic slices. A. All cultures were treated as in the displayed diagram; slices were pretreated with CX614 (50 μM, 1 day), transferred to fresh medium for 1 day, and then treated with MPP+ (20 μM) for 2 days. Tissues and media were collected for western blot analysis of BDNF (B), TH (C), and SBDP (D) contents and for LDH activity (E), respectively. B. BDNF western blot (upper panel) and quantification (lower panel) of total BDNF levels. C. Tyrosine hydroxylase (TH) western blot (upper panel) and quantification (lower panel) of total TH levels. D. Quantification of SBDP accumulation. E. LDH activity in media collected at the end of treatment (d4). Results are expressed as percent of control and are means ± SEM of 4 (B–D) and 6 (E) independent experiments. Statistics calculated with unpaired Student’s _t_-test (B–D) and with ANOVA followed by Tukey’s test (E); *, p < 0.05; **, p < 0.01; ns, not significant.
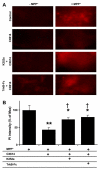
Fig. 6
Effects of TrkB-Fc and K252a on CX614-mediated protection against MPP+-induced toxicity in cultured mesencephalic slices. Slices were treated as in Fig. 5A, except that K252a (0.5 μM) of TrkB-Fc (0.3 μg/ml) treatments were initiated 30 min and 1 h, respectively, before CX614 (50 μM). Propidium iodide was added during the last day of the experiment. A. Fluorescent photomicrographs of representative fields of the substantia nigra from mesencephalic slices treated as indicated. Images were taken within 30 min of each other at the termination of all treatments. B. Quantification of data from the indicated experimental conditions (see _Section_2). Results are expressed as percent of maximum from 2 independent experiments with 8–12 mesencephalic slices in each group, and are means ± SEM. The results indicate significant differences between MPP+- and CX614 + MPP+-treated slices (**, p < 0.01; unpaired Student’s _t_-test) and between MPP+-, and CX614 + K252a + MPP+- or CX614 + TrkB-Fc + MPP+-treated cultures (*, p < 0.05; unpaired Student’s _t_-test). Results from CX614 + MPP+-treated cultures are also significantly different from CX614 + K252a + MPP+- and CX614 + TrkB-Fc + MPP+-treated cultures (†, p < 0.05; unpaired Student’s _t_-test).
Similar articles
- Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation.
Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Jourdi H, et al. J Neurosci. 2009 Jul 8;29(27):8688-97. doi: 10.1523/JNEUROSCI.6078-08.2009. J Neurosci. 2009. PMID: 19587275 Free PMC article. - Chronic elevation of brain-derived neurotrophic factor by ampakines.
Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Lauterborn JC, et al. J Pharmacol Exp Ther. 2003 Oct;307(1):297-305. doi: 10.1124/jpet.103.053694. Epub 2003 Jul 31. J Pharmacol Exp Ther. 2003. PMID: 12893840 - Ampakines cause sustained increases in brain-derived neurotrophic factor signaling at excitatory synapses without changes in AMPA receptor subunit expression.
Lauterborn JC, Pineda E, Chen LY, Ramirez EA, Lynch G, Gall CM. Lauterborn JC, et al. Neuroscience. 2009 Mar 3;159(1):283-95. doi: 10.1016/j.neuroscience.2008.12.018. Epub 2008 Dec 24. Neuroscience. 2009. PMID: 19141314 Free PMC article. - Possible protective action of neurotrophic factors and natural compounds against common neurodegenerative diseases.
Numakawa T. Numakawa T. Neural Regen Res. 2014 Aug 15;9(16):1506-8. doi: 10.4103/1673-5374.139474. Neural Regen Res. 2014. PMID: 25317165 Free PMC article. Review.
Cited by
- Effects of Agmatine on Depressive-Like Behavior Induced by Intracerebroventricular Administration of 1-Methyl-4-phenylpyridinium (MPP(+)).
Moretti M, Neis VB, Matheus FC, Cunha MP, Rosa PB, Ribeiro CM, Rodrigues AL, Prediger RD. Moretti M, et al. Neurotox Res. 2015 Oct;28(3):222-31. doi: 10.1007/s12640-015-9540-1. Epub 2015 Jul 9. Neurotox Res. 2015. PMID: 26156429 - Equipotent inhibition of fatty acid amide hydrolase and monoacylglycerol lipase - dual targets of the endocannabinoid system to protect against seizure pathology.
Naidoo V, Karanian DA, Vadivel SK, Locklear JR, Wood JT, Nasr M, Quizon PM, Graves EE, Shukla V, Makriyannis A, Bahr BA. Naidoo V, et al. Neurotherapeutics. 2012 Oct;9(4):801-13. doi: 10.1007/s13311-011-0100-y. Neurotherapeutics. 2012. PMID: 22270809 Free PMC article. - Brain fatty acid and transcriptome profiles of pig fed diets with different levels of soybean oil.
da Silva BP, Fanalli SL, Gomes JD, de Almeida VV, Fukumasu H, Freitas FAO, Moreira GCM, Silva-Vignato B, Reecy JM, Koltes JE, Koltes D, de Carvalho Balieiro JC, de Alencar SM, da Silva JPM, Coutinho LL, Afonso J, Regitano LCA, Mourão GB, Luchiari Filho A, Cesar ASM. da Silva BP, et al. BMC Genomics. 2023 Feb 28;24(1):91. doi: 10.1186/s12864-023-09188-6. BMC Genomics. 2023. PMID: 36855067 Free PMC article. - Adaptive modifications in the calpain/calpastatin system in brain cells after persistent alteration in Ca2+ homeostasis.
Stifanese R, Averna M, De Tullio R, Pedrazzi M, Beccaria F, Salamino F, Milanese M, Bonanno G, Pontremoli S, Melloni E. Stifanese R, et al. J Biol Chem. 2010 Jan 1;285(1):631-43. doi: 10.1074/jbc.M109.031674. Epub 2009 Oct 30. J Biol Chem. 2010. PMID: 19880516 Free PMC article. - Expression of full-length and truncated trkB in human striatum and substantia nigra neurons: implications for Parkinson's disease.
Fenner ME, Achim CL, Fenner BM. Fenner ME, et al. J Mol Histol. 2014 Jun;45(3):349-61. doi: 10.1007/s10735-013-9562-z. Epub 2013 Dec 29. J Mol Histol. 2014. PMID: 24374887
References
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr. Drug Targets. 2007;8(5):583–602. - PubMed
- Bi X, Chen J, Baudry M. Developmental changes in calpain activity, GluR1 receptors and in the effect of kainic acid treatment in rat brain. Neuroscience. 1997;81(4):1123–1135. - PubMed
- Blythe SN, Atherton JF, Bevan MD. Synaptic activation of dendritic AMPA and NMDA receptors generates transient high-frequency firing in substantia nigra dopamine neurons in vitro. J. Neurophysiol. 2007;97(4):2837–2850. - PubMed
- Chatha BT, Bernard V, Streit P, Bolam JP. Synaptic localization of ionotropic glutamate receptors in the rat substantia nigra. Neuroscience. 2000;101(4):1037–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS045260/NS/NINDS NIH HHS/United States
- R01 NS048521/NS/NINDS NIH HHS/United States
- NS048521-02/NS/NINDS NIH HHS/United States
- P01NS045260-01/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous