Developmentally programmed endoreduplication in animals - PubMed (original) (raw)
Developmentally programmed endoreduplication in animals
Zakir Ullah et al. Cell Cycle. 2009.
Abstract
Development of a fertilized egg into an adult human requires trillions of cell divisions, the vast majority of which duplicate their genome once and only once. Nevertheless, trophoblast giant cells and megakaryocytes in mammals circumvent this rule by duplicating their genome multiple times without undergoing cell division, a process generally referred to as 'endoreduplication'. In contrast, arthropods such as Drosophila endoreduplicate their genome in most larval tissues, as well as in many adult tissues. Endoreduplication requires that cells prevent entrance into or completion of mitosis and cytokinesis under conditions that permit assembly of prereplication complexes. In addition, cells must prevent induction of apoptosis in response to incomplete DNA replication or DNA damage that may occur during the ensuing sequence of 'endocycles'. Thus, developmentally regulated endoreduplication results in terminal cell differentiation. Recent progress has revealed both differences and similarities in the mechanisms employed by flies and mammals to change from mitotic cell cycles to 'endocycles'. The critical step, however, appears to be switching from a CDK-dependent form of the anaphase promoting complex (APC) to one that functions only in the absence of CDK activity.
Figures
Figure 1
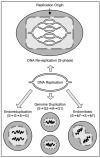
Mechanisms that generate polyploidy. Bi-directional DNA replication during mitotic cell cycles results in two diploid mononucleated daughter cells with each nucleus containing two copies of each homologous chromatid (only one is illustrated). Alternatively, cells can become polyploidy by duplicating their genome a second time, either by not passing through mitosis and cytokinesis (endoreduplication), or by passing through mitosis in the absence of cytokinesis (endomitosis), indicated by M*. Multiple rounds of endoreduplication (endocycles) produce mononucleated giant cells. Multiple rounds of endomitosis produce multilobulated nuclei in giant cells that sometimes appear as multinucleated giant cells. Mononucleated giant cells also can result from aberrant re-replication of DNA during a single mitotic cell cycle S-phase.
Figure 2
Developmentally programmed endoreduplication in trophoblast giant cells (TG) can be distinguished from DNA re-replication induced by suppression of geminin expression in cancer cells (SW480-Gmnn3) by FACS analysis.
Figure 3
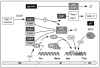
Regulation of preRC assembly during the M→G1 transition in mammals. The same mechanisms exist in flies, although the nomenclature differs (Table 1). Symbols: (→) indicates that one form of the indicated protein converts into another form. (+→) indicates activation of the target protein. (→Ø) indicates protein is degraded by the 26S proteasome. Prophase (Pro), Metaphase (Met), Anaphase (Ana) and Telophase (Telo).
Figure 4
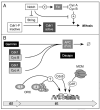
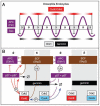
Transition to endocycles in Drosophila. (A) In follicle cells, the Notch signaling pathway blocks entrance into mitosis by inhibiting transcription of string, the gene encoding the protein phosphatase required for activation of Cdk1, as well as the genes encoding mitotic cyclins, and activates transcription of fzr, the gene required for APC(Fzr) activity. (B) In cells programmed for endoreduplication, inhibition of Cdk1 activity results in premature assembly of APC(Fzr), a ubiquitin ligase that operates in the absence of CDK activity and that targets Cyclins A and B as well as Geminin for degradation by the 26S proteasome (→Ø). Thus, a feedback loop exists that reinforces inhibition of Cdk1 activity triggered by endocycle entry. In the absence of Geminin and CDK-dependent protein phosphorylation, preRC assembly occurs as though cells had entered G1-phase. In Drosophila, ORC is essential for mitotic cell cycles in both the central nervous system and imaginal discs, and for gene amplification in ovarian follicle cells. Remarkably, however, ORC does not appear to be required for endoreduplication in the salivary gland. Since Double-parked (Dup) and the MCM DNA helicase are both required for endoreduplication under these conditions, an alternative mechanism for initiation of DNA replication may exist in some cell types. Symbols are as in Figure 3. Inhibition of the target protein is indicated by (⊥).
Figure 5
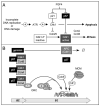
Transition to endocycles in mammals. (A) FGF4 deprivation of mouse trophoblast stem cells induces expression of both p21 and p57, two CDK-specific inhibitors unique to mammals. p21 suppresses expression of Chk1 by an unknown mechanism, and p57 inhibits Cdk1 activity by binding to Cdk1:cyclin complexes. (B) p57 inhibition of Cdk1 activity triggers the transition from mitotic cell cycles to endocycles. Symbols are as in Figure 3. Inhibition of the target protein is indicated by (⊥).
Figure 6
Sustaining endocycles in flies and mammals. (A) In Drosophila, oscillation in APC(Fzr) activity and Dacapo protein is inversely related to oscillation of Cyclin E:Cdk2 activity, an inhibitor of APC(Fzr) activity. Oscillation of APC(Fzr) activity, in turn, imposes oscillations on the cellular levels of proteins that it targets for ubiquitination and subsequent degradation, such as Geminin, Cyclin A, Cyclin B and Orc1. (B) Based on the properties of cell cycle proteins in flies and mammals, endocyles can be represented as a sequence of feed-back loops. Indicated are inhibition (⊥) by CDK-dependent phosphorylation (P) and by ubiquitination (Ub), resulting in degradation by the 26S proteasome (→Ø). Targets for SCF(Skp2) require prior CDK phosphorylation (P→).
Similar articles
- Endoreplication.
Zielke N, Edgar BA, DePamphilis ML. Zielke N, et al. Cold Spring Harb Perspect Biol. 2013 Jan 1;5(1):a012948. doi: 10.1101/cshperspect.a012948. Cold Spring Harb Perspect Biol. 2013. PMID: 23284048 Free PMC article. Review. - Role of cell cycling and polyploidy in placental trophoblast of different mammalian species.
Zybina TG, Zybina EV. Zybina TG, et al. Reprod Domest Anim. 2020 Aug;55(8):895-904. doi: 10.1111/rda.13732. Epub 2020 Jun 13. Reprod Domest Anim. 2020. PMID: 32470192 Review. - Visualizing developmentally programmed endoreplication in mammals using ubiquitin oscillators.
Sakaue-Sawano A, Hoshida T, Yo M, Takahashi R, Ohtawa K, Arai T, Takahashi E, Noda S, Miyoshi H, Miyawaki A. Sakaue-Sawano A, et al. Development. 2013 Nov;140(22):4624-32. doi: 10.1242/dev.099226. Epub 2013 Oct 23. Development. 2013. PMID: 24154524 - Reprogramming the cell cycle for endoreduplication in rodent trophoblast cells.
MacAuley A, Cross JC, Werb Z. MacAuley A, et al. Mol Biol Cell. 1998 Apr;9(4):795-807. doi: 10.1091/mbc.9.4.795. Mol Biol Cell. 1998. PMID: 9529378 Free PMC article. - Endoreduplication and activation of the anaphase-promoting complex during symbiotic cell development.
Kondorosi E, Kondorosi A. Kondorosi E, et al. FEBS Lett. 2004 Jun 1;567(1):152-7. doi: 10.1016/j.febslet.2004.04.075. FEBS Lett. 2004. PMID: 15165909 Review.
Cited by
- Physiological DNA damage promotes functional endoreplication of mammary gland alveolar cells during lactation.
Molinuevo R, Menendez J, Cadle K, Ariqat N, Choy MK, Lagousis C, Thomas G, Strietzel C, Bubolz JW, Hinck L. Molinuevo R, et al. Nat Commun. 2024 Apr 17;15(1):3288. doi: 10.1038/s41467-024-47668-9. Nat Commun. 2024. PMID: 38627401 Free PMC article. - Testis exposure to unopposed/elevated activin A in utero affects somatic and germ cells and alters steroid levels mimicking phthalate exposure.
Whiley PAF, Luu MCM, O'Donnell L, Handelsman DJ, Loveland KL. Whiley PAF, et al. Front Endocrinol (Lausanne). 2023 Sep 1;14:1234712. doi: 10.3389/fendo.2023.1234712. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37727456 Free PMC article. - The antagonistic relationship between apoptosis and polyploidy in development and cancer.
Herriage HC, Huang YT, Calvi BR. Herriage HC, et al. Semin Cell Dev Biol. 2024 Mar 15;156:35-43. doi: 10.1016/j.semcdb.2023.05.009. Epub 2023 Jun 16. Semin Cell Dev Biol. 2024. PMID: 37331841 Review. - Myc promotes polyploidy in murine trophoblast cells and suppresses senescence.
Singh VP, Hassan H, Deng F, Tsuchiya D, McKinney S, Ferro K, Gerton JL. Singh VP, et al. Development. 2023 Jun 1;150(11):dev201581. doi: 10.1242/dev.201581. Epub 2023 Jun 6. Development. 2023. PMID: 37278344 Free PMC article. - Transcriptomic Drivers of Differentiation, Maturation, and Polyploidy in Human Extravillous Trophoblast.
Morey R, Farah O, Kallol S, Requena DF, Meads M, Moretto-Zita M, Soncin F, Laurent LC, Parast MM. Morey R, et al. Front Cell Dev Biol. 2021 Sep 3;9:702046. doi: 10.3389/fcell.2021.702046. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34540826 Free PMC article.
References
- Saxena S, Dutta A. Geminin-Cdt1 balance is critical for genetic stability. Mut Res. 2005;569:111–21. - PubMed
- Smith AV, Orr-Weaver TL. The regulation of the cell cycle during Drosophila embryogenesis: the transition to polyteny. Development. 1991;112:997–1008. - PubMed
- Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice—a review. Placenta. 2005;26:3–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases