The transcriptional network that controls growth arrest and differentiation in a human myeloid leukemia cell line - PubMed (original) (raw)
doi: 10.1038/ng.375. Epub 2009 Apr 19.
Harukazu Suzuki, Alistair R R Forrest, Erik van Nimwegen, Carsten O Daub, Piotr J Balwierz, Katharine M Irvine, Timo Lassmann, Timothy Ravasi, Yuki Hasegawa, Michiel J L de Hoon, Shintaro Katayama, Kate Schroder, Piero Carninci, Yasuhiro Tomaru, Mutsumi Kanamori-Katayama, Atsutaka Kubosaki, Altuna Akalin, Yoshinari Ando, Erik Arner, Maki Asada, Hiroshi Asahara, Timothy Bailey, Vladimir B Bajic, Denis Bauer, Anthony G Beckhouse, Nicolas Bertin, Johan Björkegren, Frank Brombacher, Erika Bulger, Alistair M Chalk, Joe Chiba, Nicole Cloonan, Adam Dawe, Josee Dostie, Pär G Engström, Magbubah Essack, Geoffrey J Faulkner, J Lynn Fink, David Fredman, Ko Fujimori, Masaaki Furuno, Takashi Gojobori, Julian Gough, Sean M Grimmond, Mika Gustafsson, Megumi Hashimoto, Takehiro Hashimoto, Mariko Hatakeyama, Susanne Heinzel, Winston Hide, Oliver Hofmann, Michael Hörnquist, Lukasz Huminiecki, Kazuho Ikeo, Naoko Imamoto, Satoshi Inoue, Yusuke Inoue, Ryoko Ishihara, Takao Iwayanagi, Anders Jacobsen, Mandeep Kaur, Hideya Kawaji, Markus C Kerr, Ryuichiro Kimura, Syuhei Kimura, Yasumasa Kimura, Hiroaki Kitano, Hisashi Koga, Toshio Kojima, Shinji Kondo, Takeshi Konno, Anders Krogh, Adele Kruger, Ajit Kumar, Boris Lenhard, Andreas Lennartsson, Morten Lindow, Marina Lizio, Cameron Macpherson, Norihiro Maeda, Christopher A Maher, Monique Maqungo, Jessica Mar, Nicholas A Matigian, Hideo Matsuda, John S Mattick, Stuart Meier, Sei Miyamoto, Etsuko Miyamoto-Sato, Kazuhiko Nakabayashi, Yutaka Nakachi, Mika Nakano, Sanne Nygaard, Toshitsugu Okayama, Yasushi Okazaki, Haruka Okuda-Yabukami, Valerio Orlando, Jun Otomo, Mikhail Pachkov, Nikolai Petrovsky, Charles Plessy, John Quackenbush, Aleksandar Radovanovic, Michael Rehli, Rintaro Saito, Albin Sandelin, Sebastian Schmeier, Christian Schönbach, Ariel S Schwartz, Colin A Semple, Miho Sera, Jessica Severin, Katsuhiko Shirahige, Cas Simons, George St Laurent, Masanori Suzuki, Takahiro Suzuki, Matthew J Sweet, Ryan J Taft, Shizu Takeda, Yoichi Takenaka, Kai Tan, Martin S Taylor, Rohan D Teasdale, Jesper Tegnér, Sarah Teichmann, Eivind Valen, Claes Wahlestedt, Kazunori Waki, Andrew Waterhouse, Christine A Wells, Ole Winther, Linda Wu, Kazumi Yamaguchi, Hiroshi Yanagawa, Jun Yasuda, Mihaela Zavolan, David A Hume; Riken Omics Science Center; Takahiro Arakawa, Shiro Fukuda, Kengo Imamura, Chikatoshi Kai, Ai Kaiho, Tsugumi Kawashima, Chika Kawazu, Yayoi Kitazume, Miki Kojima, Hisashi Miura, Kayoko Murakami, Mitsuyoshi Murata, Noriko Ninomiya, Hiromi Nishiyori, Shohei Noma, Chihiro Ogawa, Takuma Sano, Christophe Simon, Michihira Tagami, Yukari Takahashi, Jun Kawai, Yoshihide Hayashizaki
Collaborators, Affiliations
- PMID: 19377474
- PMCID: PMC6711855
- DOI: 10.1038/ng.375
The transcriptional network that controls growth arrest and differentiation in a human myeloid leukemia cell line
FANTOM Consortium et al. Nat Genet. 2009 May.
Abstract
Using deep sequencing (deepCAGE), the FANTOM4 study measured the genome-wide dynamics of transcription-start-site usage in the human monocytic cell line THP-1 throughout a time course of growth arrest and differentiation. Modeling the expression dynamics in terms of predicted cis-regulatory sites, we identified the key transcription regulators, their time-dependent activities and target genes. Systematic siRNA knockdown of 52 transcription factors confirmed the roles of individual factors in the regulatory network. Our results indicate that cellular states are constrained by complex networks involving both positive and negative regulatory interactions among substantial numbers of transcription factors and that no single transcription factor is both necessary and sufficient to drive the differentiation process.
Figures
Figure 1
Motif Activity Response Analysis (MARA). (a) CAGE tags are mapped to the human genome and their expression is normalized; vertical lines represent TSS positions, and their height is proportional to the normalized expression. (b) Mapped tags are clustered into promoters on the basis of their relative expression, and neighboring promoters are joined into promoter regions. (c) A window of −300 to +100 flanking each promoter region is extracted, multiply aligned and the MotEvo algorithm is used to predict binding sites for known motifs. (d–f) Observed expression of all promoters (d) and predicted site-counts (e) are used to infer motif activities (f). (g) The statistical significance of the regulatory edge from motif to promoter is calculated based on correlation of the promoter expression and motif activity profiles.
Figure 2
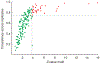
Statistical significance and consistency across replicates of the inferred motif activity profiles. Each dot corresponds to a motif. The significance of each motif in explaining the observed expression variation is quantified by the z value of its activity profile (horizontal axis, see Methods). The consistency of the inferred activity profile of each motif is quantified by the fraction of the variance (FOV) in the activity profile across all six replicates (three biological replicates for both CAGE and Illumina), which is reproduced in each replicate (vertical axis, see Methods).
Figure 3
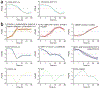
Inferred time-dependent activities of the key regulatory motifs. (a) The time-dependent activity profile of the E2F1-5 regulatory motif as inferred from CAGE (left) and microarray (right) data. The three biological replicates are shown in red, blue and green. (b) The 30 most significant motifs with consistent activity profiles across all replicates (CAGE and microarray) were clustered into nine sets of motifs with similar dynamics. Each panel shows the activity of the members of the cluster (colored curves), the names of motifs contributing and the cluster average activity profile (black).
Figure 4
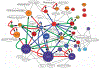
Predicted core regulatory network of the 30 core motifs. An edge X→Y is drawn whenever the promoter of at least one of the TFs associated with motif Y has a predicted regulatory edge for motif X (z value ≥1.5) and the edge has independent experimental support. The color of each node reflects its cluster membership and the size of the node reflects the significance of the motif. Edges confirmed in the literature, by ChIP or by siRNA are shown in red, blue and green, respectively. In cases where there are multiple lines of support only one evidence type is shown. Supplementary Table 5 shows all predicted edges and their experimental support. GO terms significantly enriched among target genes are shown as white nodes with black edges. FOS/JUN (FOS,B,L1_JUNB,D), CREB (ATF5_CREB3), GABPA (ELK1,4_GABPA,B2).
Figure 5
Validation of predicted target promoter sets using siRNA knockdowns. (a) Difference in the average log expression ratio upon knockdown between predicted target promoters and predicted nontargets (vertical axis) as a function of the _z_-value cut-off on target prediction (horizontal axis, more stringent cut-offs are on the right) for knockdown of the TF genes MYB (red), SNAI3 (orange), RUNX1 (green) and EGR1 (light blue). (b) As in a but now for knockdown of SPI1 followed by 1 h without treatment (light blue), 24 h without treatment (dark blue), 1 h of PMA treatment (orange) and 24 h of PMA treatment (red). All straight lines are linear regression fits. (c) Pearson correlation coefficients between the average log expression ratio difference of targets and nontargets and the cut-off on target predictions (horizontal axis). Red bars indicate correlation coefficients larger than 0.75 in absolute value; green bars, absolute values between 0.5 and 0.75; and blue bars, less than 0.5. (d) Significance (z value) of the difference in log expression ratio between predicted targets and nontargets (cut-off z = 1.5) for all 28 TFs associated with a motif, measured as a z value (number of standard errors). Red bars correspond to significant changes, that is, greater than two standard errors; green bars, changes between 1 and 2 standard errors; and blue bars, changes less than 1 standard error. siRNA knockdowns were carried out in biological triplicate and knockdown was assessed by qRT-PCR (Supplementary Table 7).
Figure 6
Most significant motif activity changes (as measured by z value, red bars) for four TF gene knockdowns that induce motif activity changes that have a differentiative overlap with the PMA time course of more than 50%. The corresponding motif activity changes observed in the PMA time course are shown as gray bars.
Similar articles
- Building promoter aware transcriptional regulatory networks using siRNA perturbation and deepCAGE.
Vitezic M, Lassmann T, Forrest AR, Suzuki M, Tomaru Y, Kawai J, Carninci P, Suzuki H, Hayashizaki Y, Daub CO. Vitezic M, et al. Nucleic Acids Res. 2010 Dec;38(22):8141-8. doi: 10.1093/nar/gkq729. Epub 2010 Aug 19. Nucleic Acids Res. 2010. PMID: 20724440 Free PMC article. - FANTOM4 EdgeExpressDB: an integrated database of promoters, genes, microRNAs, expression dynamics and regulatory interactions.
Severin J, Waterhouse AM, Kawaji H, Lassmann T, van Nimwegen E, Balwierz PJ, de Hoon MJ, Hume DA, Carninci P, Hayashizaki Y, Suzuki H, Daub CO, Forrest AR. Severin J, et al. Genome Biol. 2009;10(4):R39. doi: 10.1186/gb-2009-10-4-r39. Epub 2009 Apr 19. Genome Biol. 2009. PMID: 19374773 Free PMC article. - Regulatory interdependence of myeloid transcription factors revealed by Matrix RNAi analysis.
Tomaru Y, Simon C, Forrest AR, Miura H, Kubosaki A, Hayashizaki Y, Suzuki M. Tomaru Y, et al. Genome Biol. 2009;10(11):R121. doi: 10.1186/gb-2009-10-11-r121. Epub 2009 Nov 2. Genome Biol. 2009. PMID: 19883503 Free PMC article. - Differentiation primary response genes and proto-oncogenes as positive and negative regulators of terminal hematopoietic cell differentiation.
Liebermann DA, Hoffman B. Liebermann DA, et al. Stem Cells. 1994 Jul;12(4):352-69. doi: 10.1002/stem.5530120402. Stem Cells. 1994. PMID: 7951003 Review. - [Analysis of the transcriptional regulatory network in the model organisms: yeast and sea urchin].
Takamatsu K, Takeda T. Takamatsu K, et al. Tanpakushitsu Kakusan Koso. 2004 Dec;49(17 Suppl):2742-50. Tanpakushitsu Kakusan Koso. 2004. PMID: 15669249 Review. Japanese. No abstract available.
Cited by
- Identification of c-Myb Target Genes in K562 Cells Reveals a Role for c-Myb as a Master Regulator.
Lorenzo PI, Brendeford EM, Gilfillan S, Gavrilov AA, Leedsak M, Razin SV, Eskeland R, Sæther T, Gabrielsen OS. Lorenzo PI, et al. Genes Cancer. 2011 Aug;2(8):805-17. doi: 10.1177/1947601911428224. Genes Cancer. 2011. PMID: 22393465 Free PMC article. - DDBJ launches a new archive database with analytical tools for next-generation sequence data.
Kaminuma E, Mashima J, Kodama Y, Gojobori T, Ogasawara O, Okubo K, Takagi T, Nakamura Y. Kaminuma E, et al. Nucleic Acids Res. 2010 Jan;38(Database issue):D33-8. doi: 10.1093/nar/gkp847. Epub 2009 Oct 22. Nucleic Acids Res. 2010. PMID: 19850725 Free PMC article. - DBTSS provides a tissue specific dynamic view of Transcription Start Sites.
Yamashita R, Wakaguri H, Sugano S, Suzuki Y, Nakai K. Yamashita R, et al. Nucleic Acids Res. 2010 Jan;38(Database issue):D98-104. doi: 10.1093/nar/gkp1017. Epub 2009 Nov 12. Nucleic Acids Res. 2010. PMID: 19910371 Free PMC article. - Development of a high-throughput method for the systematic identification of human proteins nuclear translocation potential.
Hoat TX, Bertin N, Ninomiya N, Fukuda S, Usui K, Kawai J, Hayashizaki Y, Suzuki H. Hoat TX, et al. BMC Cell Biol. 2009 Sep 22;10:69. doi: 10.1186/1471-2121-10-69. BMC Cell Biol. 2009. PMID: 19772597 Free PMC article. - Dynamic modelling of microRNA regulation during mesenchymal stem cell differentiation.
Weber M, Sotoca AM, Kupfer P, Guthke R, van Zoelen EJ. Weber M, et al. BMC Syst Biol. 2013 Nov 12;7:124. doi: 10.1186/1752-0509-7-124. BMC Syst Biol. 2013. PMID: 24219887 Free PMC article.
References
- Tsuchiya S et al. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 42, 1530–1536 (1982). - PubMed
- Abrink M, Gobl AE, Huang R, Nilsson K & Hellman L Human cell lines U-937, THP-1 and Mono Mac 6 represent relatively immature cells of the monocyte-macrophage cell lineage. Leukemia 8, 1579–1584 (1994). - PubMed
- Beer MA & Tavazoie S Predicting gene expression from sequence. Cell 117, 185–198 (2004). - PubMed
- Segal E et al. Module networks: identifying regulatory modules and their condition-specific regulators from gene expression data. Nat. Genet 34, 166–176 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- TCR05001/TI_/Telethon/Italy
- WT_/Wellcome Trust/United Kingdom
- MC_U105161047/MRC_/Medical Research Council/United Kingdom
- R01 LM010129/LM/NLM NIH HHS/United States
- R01 HL111759/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials