Valosin-containing protein disease: inclusion body myopathy with Paget's disease of the bone and fronto-temporal dementia - PubMed (original) (raw)
Review
Valosin-containing protein disease: inclusion body myopathy with Paget's disease of the bone and fronto-temporal dementia
Conrad C Weihl et al. Neuromuscul Disord. 2009 May.
Abstract
Mutations in valosin-containing protein (VCP) cause inclusion body myopathy (IBM) associated with Paget's disease of the bone (PDB) and fronto-temporal dementia (FTD) or IBMPFD. Although IBMPFD is a multisystem disorder, muscle weakness is the presenting symptom in greater than half of patients and an isolated symptom in 30%. Patients with the full spectrum of the disease make up only 12% of those affected; therefore it is important to consider and recognize IBMPFD in a neuromuscular clinic. The current review describes the skeletal muscle phenotype and common muscle histochemical features in IBMPFD. In addition to myopathic features; vacuolar changes and tubulofilamentous inclusions are found in a subset of patients. The most consistent findings are VCP, ubiquitin and TAR DNA-binding protein 43 (TDP-43) positive inclusions. VCP is a ubiquitously expressed multifunctional protein that is a member of the AAA+ (ATPase associated with various activities) protein family. It has been implicated in multiple cellular functions ranging from organelle biogenesis to protein degradation. Although the role of VCP in skeletal muscle is currently unknown, it is clear that VCP mutations lead to the accumulation of ubiquitinated inclusions and protein aggregates in patient tissue, transgenic animals and in vitro systems. We suggest that IBMPFD is novel type of protein surplus myopathy. Instead of accumulating a poorly degraded and aggregated mutant protein as seen in some myofibrillar and nemaline myopathies, VCP mutations disrupt its normal role in protein homeostasis resulting in the accumulation of ubiquitinated and aggregated proteins that are deleterious to skeletal muscle.
Figures
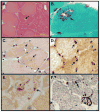
Figure 1
Histochemical and immunohistochemical stains of IBMPFD patient muscle biopsies. A) hematoxylin and eosin demonstrating large regions of grouped atrophy with smaller fibers containing rimmed vacuole, B) A fiber with a prominent rimmed vacuole seen with modified gomori trichrome stain, C) congo red staining demonstrating fibers with vacuoles and basophilic debris. D) Immunostaining with an anti-ubiquitin antibody (FK2) demonstrates large inclusions that are myonuclear and subsarcolemmal. E) A vacuolated fiber with VCP inclusions. F) TDP-43 immunostaining sarcoplasmic inclusions. Closed arrows highlight vacuoles and open arrows denote inclusions
Figure 2
A) Space fill diagram of a VCP hexamer (top) with each monomer colored individually and a linear diagram of a single VCP monomer showing the N-domain, L1 and L2 linkers, D1 and D2 ATPase domains and the C-terminal domain (bottom). Red space fill in hexamer represents residues that are mutated in IBMPFD. *Mutation T262A is unpublished (Spina S and Ghetti B, 2007 American Academy of Neurology Meeting).
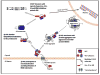
Figure 3
Proposed functions of VCP. 1) VCP participates in the retrotranslocation and subsequent ubiquitination of ERAD substrates via binding to derlin-1 and one of several ER associated E3 ubiquitin ligases such as Hrd1 or gp78. 2) VCP shuttles ubiquitinated protein substrates to the 26S proteasome via interactions with rad23. 3) VCP mediates degradation of many short-lived signaling molecules such as Unc-45b and IκB. Unc-45 is responsible for myosin folding and myofibrillogenesis and IkB mediates NFkB signal transduction. 4) In the setting of UPS dysfunction or protein misfolding, VCP associates with small ubiquitinated protein aggregates and facilitates their sequestration into “aggresomes” via direct interactions with HDAC6. Aggresomes are then degraded via the autophagosome-lysosome system.
Similar articles
- The multiple faces of valosin-containing protein-associated diseases: inclusion body myopathy with Paget's disease of bone, frontotemporal dementia, and amyotrophic lateral sclerosis.
Nalbandian A, Donkervoort S, Dec E, Badadani M, Katheria V, Rana P, Nguyen C, Mukherjee J, Caiozzo V, Martin B, Watts GD, Vesa J, Smith C, Kimonis VE. Nalbandian A, et al. J Mol Neurosci. 2011 Nov;45(3):522-31. doi: 10.1007/s12031-011-9627-y. Epub 2011 Sep 3. J Mol Neurosci. 2011. PMID: 21892620 Review. - Valosin-containing protein and the pathogenesis of frontotemporal dementia associated with inclusion body myopathy.
Guinto JB, Ritson GP, Taylor JP, Forman MS. Guinto JB, et al. Acta Neuropathol. 2007 Jul;114(1):55-61. doi: 10.1007/s00401-007-0224-7. Epub 2007 Apr 25. Acta Neuropathol. 2007. PMID: 17457594 Review. - Valosin containing protein associated fronto-temporal lobar degeneration: clinical presentation, pathologic features and pathogenesis.
Weihl CC. Weihl CC. Curr Alzheimer Res. 2011 May;8(3):252-60. doi: 10.2174/156720511795563773. Curr Alzheimer Res. 2011. PMID: 21222596 Free PMC article. Review. - Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation.
Weihl CC, Dalal S, Pestronk A, Hanson PI. Weihl CC, et al. Hum Mol Genet. 2006 Jan 15;15(2):189-99. doi: 10.1093/hmg/ddi426. Epub 2005 Dec 1. Hum Mol Genet. 2006. PMID: 16321991 - Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease.
Ju JS, Fuentealba RA, Miller SE, Jackson E, Piwnica-Worms D, Baloh RH, Weihl CC. Ju JS, et al. J Cell Biol. 2009 Dec 14;187(6):875-88. doi: 10.1083/jcb.200908115. J Cell Biol. 2009. PMID: 20008565 Free PMC article.
Cited by
- VCP regulates early tau seed amplification via specific cofactors.
Batra S, Vaquer-Alicea JI, Valdez C, Taylor SP, Manon VA, Vega AR, Kashmer OM, Kolay S, Lemoff A, Cairns NJ, White CL, Diamond MI. Batra S, et al. Res Sq [Preprint]. 2024 May 22:rs.3.rs-4307848. doi: 10.21203/rs.3.rs-4307848/v1. Res Sq. 2024. PMID: 38826306 Free PMC article. Preprint. - The new missense G376V-TDP-43 variant induces late-onset distal myopathy but not amyotrophic lateral sclerosis.
Zibold J, Lessard LER, Picard F, da Silva LG, Zadorozhna Y, Streichenberger N, Belotti E, Osseni A, Emerit A, Errazuriz-Cerda E, Michel-Calemard L, Menassa R, Coudert L, Wiessner M, Stucka R, Klopstock T, Simonetti F, Hutten S, Nonaka T, Hasegawa M, Strom TM, Bernard E, Ollagnon E, Urtizberea A, Dormann D, Petiot P, Schaeffer L, Senderek J, Leblanc P. Zibold J, et al. Brain. 2024 May 3;147(5):1768-1783. doi: 10.1093/brain/awad410. Brain. 2024. PMID: 38079474 Free PMC article. - Targeting mitochondrial Ca2+ uptake for the treatment of amyotrophic lateral sclerosis.
Zhong R, Rua MT, Wei-LaPierre L. Zhong R, et al. J Physiol. 2024 Apr;602(8):1519-1549. doi: 10.1113/JP284143. Epub 2023 Nov 27. J Physiol. 2024. PMID: 38010626 Review. - Mitochondria, a Key Target in Amyotrophic Lateral Sclerosis Pathogenesis.
Genin EC, Abou-Ali M, Paquis-Flucklinger V. Genin EC, et al. Genes (Basel). 2023 Oct 24;14(11):1981. doi: 10.3390/genes14111981. Genes (Basel). 2023. PMID: 38002924 Free PMC article. Review.
References
- Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–81. - PubMed
- Kimonis VE, Watts GD. Autosomal dominant inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Alzheimer Dis Assoc Disord. 2005;19 1:S44–7. - PubMed
- Guyant-Marechal L, Laquerriere A, Duyckaerts C, et al. Valosin-containing protein gene mutations: clinical and neuropathologic features. Neurology. 2006;67:644–51. - PubMed
- Haubenberger D, Bittner RE, Rauch-Shorny S, et al. Inclusion body myopathy and Paget disease is linked to a novel mutation in the VCP gene. Neurology. 2005;65:1304–5. - PubMed
- Hubbers CU, Clemen CS, Kesper K, et al. Pathological consequences of VCP mutations on human striated muscle. Brain. 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 AG026271-01/AG/NIA NIH HHS/United States
- R01 AG031867-01A1/AG/NIA NIH HHS/United States
- K08 AG026271/AG/NIA NIH HHS/United States
- 5K08 AG026271/AG/NIA NIH HHS/United States
- 1R01 AG031867/AG/NIA NIH HHS/United States
- R01 AG031867/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous