Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta - PubMed (original) (raw)
Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta
Galina A Gusarova et al. Mol Cell Biol. 2009 Jul.
Abstract
Hypoxia promotes Na,K-ATPase endocytosis via protein kinase C zeta (PKC zeta)-mediated phosphorylation of the Na,K-ATPase alpha subunit. Here, we report that hypoxia leads to the phosphorylation of 5'-AMP-activated protein kinase (AMPK) at Thr172 in rat alveolar epithelial cells. The overexpression of a dominant-negative AMPK alpha subunit (AMPK-DN) construct prevented the hypoxia-induced endocytosis of Na,K-ATPase. The overexpression of the reactive oxygen species (ROS) scavenger catalase prevented hypoxia-induced AMPK activation. Moreover, hypoxia failed to activate AMPK in mitochondrion-deficient rho(0)-A549 cells, suggesting that mitochondrial ROS play an essential role in hypoxia-induced AMPK activation. Hypoxia-induced PKC zeta translocation to the plasma membrane and phosphorylation at Thr410 were prevented by the pharmacological inhibition of AMPK or by the overexpression of the AMPK-DN construct. We found that AMPK alpha phosphorylates PKC zeta on residue Thr410 within the PKC zeta activation loop. Importantly, the activation of AMPK alpha was necessary for hypoxia-induced AMPK-PKC zeta binding in alveolar epithelial cells. The overexpression of T410A mutant PKC zeta prevented hypoxia-induced Na,K-ATPase endocytosis, confirming that PKC zeta Thr410 phosphorylation is essential for this process. PKC zeta activation by AMPK is isoform specific, as small interfering RNA targeting the alpha1 but not the alpha2 catalytic subunit prevented PKC zeta activation. Accordingly, we provide the first evidence that hypoxia-generated mitochondrial ROS lead to the activation of the AMPK alpha1 isoform, which binds and directly phosphorylates PKC zeta at Thr410, thereby promoting Na,K-ATPase endocytosis.
Figures
FIG. 1.
Hypoxia activates AMPK in ATII cells. (A) ATII cells were exposed to 21% O2 (white bar) or 1.5% O2 (gray bars) for 2.5 to 30 min, and the levels of AMPK phosphorylated at Thr172 (pAMPK α) and ACC phosphorylated at Ser79 (pACC), as well as the total amount of AMPK α, were measured by Western blot analysis. The graph represents the pAMPK/AMPK ratios. Representative Western blots analyzing pAMPK α, pACC, and total AMPK α are shown. (B) ATII cells were exposed to 21% O2 (white bar) or 3% O2 (gray bars) for 5 to 60 min, and the levels of pAMPK α and pACC and the total amount of AMPK α were determined as described above. The graph represents the pAMPK/AMPK ratios. Representative Western blots analyzing pAMPK α, pACC, and total AMPK α are shown. Values are expressed as means ± SEM (n = 4). *, P < 0.05; **, P < 0.01.
FIG. 2.
AMPK activation is required for hypoxia-induced Na,K-ATPase endocytosis, independent of changes in intracellular Na+ concentrations. (A) ATII cells were infected with Ad-null (20 PFU/cell) or Ad-HA-DN AMPK-α (20 PFU/cell). After 24 h, the cells were exposed to 21 or 1.5% O2 for 60 min. Na,K-ATPase α1 subunit abundance at the plasma membrane was determined from cell surface biotinylation, followed by streptavidin pulldown and Western blot analysis with an α1 subunit-specific antibody. (B) ATII cells were infected as described above and, after 24 h, pretreated with monensin (2 μM) for 5 min and then exposed to 21 or 1.5% O2 for 60 min. Na,K-ATPase α1 subunit abundance at the plasma membrane was determined as described above. Representative Western blots analyzing the level of Na,K-ATPase subunit α1 at the plasma membrane and the level of HA-tagged AMPK expression are shown. E-cadherin was used as a loading control. Values shown are means ± SEM (n = 4). **, P < 0.01.
FIG. 3.
ROS activate AMPK during hypoxia. (A) ATII cells were infected with Ad-null or Ad-catalase (20 PFU/cell) and then exposed to 21 or 1.5% O2 for 10 min; the levels of pAMPK α and total AMPK α were determined by Western blotting as described above. Representative Western blots analyzing pAMPK α, total AMPK, and catalase are shown (n = 3). (B) ATII cells were treated with 100 μM t-H2O2 for up to 30 min; the levels of pAMPK α, pACC, and total AMPK α were determined by Western blotting. Representative Western blots are shown (n = 3). (C) WT and ρ0-A549 cells were exposed to 21 or 1.5% O2 for 10 min, and the levels of pACC and total ACC were determined by Western blotting as described above (n = 3). (D) ρ0-A549 cells were treated with 100 μM t-H2O2 or vehicle (V) for 10 min, and the levels of pACC and total ACC were determined by Western blotting. Representative Western blots are shown (n = 3).
FIG. 4.
AMPK activation promotes Na,K-ATPase endocytosis in the absence of ROS. (A) ATII cells were infected with Ad-catalase (Ad-CAT; 20 PFU/cell) and Ad-CA AMPK as described in Materials and Methods. After infection, the cells were exposed to 21 or 1.5% O2 for 60 min. Na,K-ATPase α1 abundance at the plasma membrane (PM) was determined from cell surface biotinylation, followed by streptavidin pulldown and Western blot analysis with an α1 subunit-specific antibody. Representative Western blots analyzing the expression of Na,K-ATPase α1 at the plasma membrane and in the cell lysates (TCL) as a loading control and the expression of catalase and constitutively active AMPK (CA-AMPK) in the cell lysates are shown. (B) ρ0-A549 cells were infected with Ad-null (20 PFU/cell) or Ad-CA AMPK (20 PFU/cell), and the amount of Na,K-ATPase was determined. Representative Western blots analyzing Na,K-ATPase α1 at the plasma membrane, E-cadherin as a loading control, and the expression of CA-AMPK are shown. Values are expressed as means ± SEM (n = 4). **, P < 0.01.
FIG. 5.
AMPK binds to and activates PKCζ in ATII cells during hypoxia. (A) ATII cells were infected with Ad-null (20 PFU/cell) or Ad-HA-DN AMPK-α (DN AMPK-α; 20 PFU/cell) or were pretreated with compound C (20 μM for 30 min) and exposed to 21 or 1.5% O2 for 10 min. A 1% Triton X-100-soluble membrane fraction was obtained, and PKCζ translocation was evaluated by Western blotting. Representative Western blots for PKCζ and E-cadherin (as a loading control) at the plasma membrane and for the expression levels of AMPK in the cell lysates are shown. The graph represents the PKCζ/E-cadherin ratios at the plasma membrane. Values are expressed as means ± SEM (n = 4). **, P < 0.01. (B and C) ATII cells were exposed to 21 or 1.5% O2 for 10 min. At the end of the incubation, cells were lysed and equal amounts (500 μg) of proteins were immunoprecipitated (IP) with anti-AMPK antibody or rabbit immunoglobulin G (IgG) as a control (B) or with anti-PKCζ antibody or mouse IgG (C). Immunocomplexes were analyzed by Western blotting with antibodies specific for pAMPK, total AMPK, and total PKCζ. Representative Western blots are shown. (D) PKCζ-GST fusion protein (150 ng) was incubated in the presence (+) or absence (−) of the constitutively active AMPK α1-GST fusion protein (200 ng) and [γ-32P]ATP. A representative autoradiograph of phosphorylated PKCζ and Western blots (WB) analyzing PKCζ and AMPK are shown (n = 3).
FIG. 6.
AMPK phosphorylation of PKCζ at the Thr410 residue is required for hypoxia-induced downregulation of Na,K-ATPase. (A) ATII cells were infected with Ad-null (20 PFU/cell) or Ad-HA-DN AMPK-α (DN-AMPK α; 20 PFU/cell) or pretreated with compound C (2 μM for 30 min) and exposed to 21 or 1.5% O2 for 10 min. PKCζ was immunoprecipitated from the cell lysates as described in Materials and Methods. PKCζ phosphorylated at Thr410 [pPKCζ (T410)] and the total amount of PKCζ were measured by Western blot analysis. Representative Western blots for pPKCζ (T410), PKCζ, and HA-AMPK in the cell lysates are shown (n = 4). (B) ATII cells were treated with AICAR (2 mM) for 15 min or Ad-CA AMPK (CA-AMPK; 20 PFU/cell). PKCζ was immunoprecipitated from the cell lysate as described in Materials and Methods. pPKCζ (T410), pACC as a control for AMPK activation, and the total amount of PKCζ were measured by Western blot analysis (n = 4). Representative Western blots are shown. V, vehicle. (C) COS-7 cells were transiently transfected with full-length FLAG-WT PKCζ, FLAG-T410A mutant PKCζ, or empty vector (EV); 48 h later, PKCζ was immunoprecipitated with 1 μl of FLAG antibody as described in Materials and Methods and incubated with AMPK α1-GST fusion protein (200 ng) and [γ-32P]ATP. A representative autoradiograph of the phosphorylated PKCζ and a Western blot (WB) analyzing FLAG-PKCζ are shown (n = 3). (D) COS-7 cells were transiently transfected with a vector expressing full-length FLAG-WT PKCζ or FLAG-T410A mutant PKCζ or an empty vector; 48 h later, cells were exposed to 21 or 1.5% O2 for 60 min. Na,K-ATPase α1 subunit abundance at the plasma membrane was determined from cell surface biotinylation, followed by streptavidin pulldown and Western blot analysis using specific antibodies. Representative Western blots analyzing the Na,K-ATPase α1 subunit at the plasma membrane (PM) and the Na,K-ATPase α1 subunit (as a loading control) and PKCζ in the cell lysates (TCL) are shown. Values are expressed as means ± SEM (n = 4). **, P < 0.01.
FIG. 7.
AMPK α1 is required for hypoxia-induced PKCζ activation. (A) Western blot showing the expression of AMPK α1 and α2 in rat ATII and A549+LKB1 cells and a brain cell lysate as a positive control. (B) A549+LKB1 cells were transfected with siRNA against AMPK α1 (siAMPKα1) or AMPK α2 (siAMPKα2) or with scrambled siRNA (si-scrambled); 48 h later, cells were exposed to 21 or 1.5% O2 for 10 min. PKCζ plasma membrane translocation was assessed as described above. The graph represents the PKCζ/E-cadherin ratios at the plasma membrane (PM). Representative Western blots analyzing PKCζ, E-cadherin (loading-control), AMPK α1, AMPK α2, and β-actin expression levels in the cell lysates (TCL) are shown. Values are expressed as means ± SEM (n = 4). **, P < 0.01. (C) A549+LKB1 cells were transfected with siRNA against AMPK α1 or AMPK α2 or with scrambled siRNA; 48 h later, cells were exposed to 21 or 1.5% O2 for 10 min. Na,K-ATPase was evaluated as described above. The graph represents the abundance of Na,K-ATPase at the plasma membrane. Representative Western blots analyzing Na,K-ATPase, AMPK α1 and AMPK α2, and β-actin expression levels in whole-cell lysates (TCL) are shown. Values are expressed as means ± SEM (n = 4). **, P < 0.01.
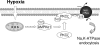
FIG. 8.
Schematic illustration of the hypoxia-induced signaling pathway leading to Na,K-ATPase downregulation. Hypoxia increases mitochondrial ROS production and leads to AMPK α1 phosphorylation. Activated AMPK directly phosphorylates PKCζ at T410, and PKCζ in turn phosphorylates the Na,K-ATPase α1 subunit at Ser18, triggering its endocytosis.
Similar articles
- Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta.
Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Dada LA, et al. J Clin Invest. 2003 Apr;111(7):1057-64. doi: 10.1172/JCI16826. J Clin Invest. 2003. PMID: 12671055 Free PMC article. - AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis.
Vadász I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Tóth PT, Lecuona E, Witters LA, Schumacker PT, Chandel NS, Seeger W, Sznajder JI. Vadász I, et al. J Clin Invest. 2008 Feb;118(2):752-62. doi: 10.1172/JCI29723. J Clin Invest. 2008. PMID: 18188452 Free PMC article. - HIF and HOIL-1L-mediated PKCζ degradation stabilizes plasma membrane Na,K-ATPase to protect against hypoxia-induced lung injury.
Magnani ND, Dada LA, Queisser MA, Brazee PL, Welch LC, Anekalla KR, Zhou G, Vagin O, Misharin AV, Budinger GRS, Iwai K, Ciechanover AJ, Sznajder JI. Magnani ND, et al. Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):E10178-E10186. doi: 10.1073/pnas.1713563114. Epub 2017 Nov 6. Proc Natl Acad Sci U S A. 2017. PMID: 29109255 Free PMC article. - Regulation of Na,K-ATPase during acute lung injury.
Lecuona E, Trejo HE, Sznajder JI. Lecuona E, et al. J Bioenerg Biomembr. 2007 Dec;39(5-6):391-5. doi: 10.1007/s10863-007-9102-1. J Bioenerg Biomembr. 2007. PMID: 17972021 Review. - Hypoxic inhibition of alveolar fluid reabsorption.
Dada LA, Sznajder JI. Dada LA, et al. Adv Exp Med Biol. 2007;618:159-68. doi: 10.1007/978-0-387-75434-5_12. Adv Exp Med Biol. 2007. PMID: 18269195 Review.
Cited by
- Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells.
Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Waypa GB, et al. Circ Res. 2010 Feb 19;106(3):526-35. doi: 10.1161/CIRCRESAHA.109.206334. Epub 2009 Dec 17. Circ Res. 2010. PMID: 20019331 Free PMC article. - Rapamycin improves satellite cells' autophagy and muscle regeneration during hypercapnia.
Balnis J, Jackson EL, Drake LA, Singer DV, Bossardi Ramos R, Singer HA, Jaitovich A. Balnis J, et al. JCI Insight. 2025 Jan 9;10(1):e182842. doi: 10.1172/jci.insight.182842. JCI Insight. 2025. PMID: 39589836 Free PMC article. - Inter-connection between mitochondria and HIFs.
Tormos KV, Chandel NS. Tormos KV, et al. J Cell Mol Med. 2010 Apr;14(4):795-804. doi: 10.1111/j.1582-4934.2010.01031.x. Epub 2010 Feb 16. J Cell Mol Med. 2010. PMID: 20158574 Free PMC article. Review. - Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation.
Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Waypa GB, et al. Am J Respir Crit Care Med. 2013 Feb 15;187(4):424-32. doi: 10.1164/rccm.201207-1294OC. Epub 2013 Jan 17. Am J Respir Crit Care Med. 2013. PMID: 23328522 Free PMC article. - Is enough oxygen too much?
Schumacker PT. Schumacker PT. Crit Care. 2010;14(4):191. doi: 10.1186/cc9201. Epub 2010 Aug 24. Crit Care. 2010. PMID: 20804573 Free PMC article.
References
- Bertorello, A. M., Y. Komarova, K. Smith, I. B. Leibiger, R. Efendiev, C. H. Pedemonte, G. Borisy, and J. I. Sznajder. 2003. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol. Biol. Cell 141149-1157. - PMC - PubMed
- Bhalla, V., N. M. Oyster, A. C. Fitch, M. A. Wijngaarden, D. Neumann, U. Schlattner, D. Pearce, and K. R. Hallows. 2006. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J. Biol. Chem. 28126159-26169. - PubMed
- Brunelle, J. K., E. L. Bell, N. M. Quesada, K. Vercauteren, V. Tiranti, M. Zeviani, R. C. Scarpulla, and N. S. Chandel. 2005. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 1409-414. - PubMed
- Carling, D., V. A. Zammit, and D. G. Hardie. 1987. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 223217-222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases