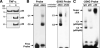A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension - PubMed (original) (raw)
. 2009 May 5;119(17):2313-22.
doi: 10.1161/CIRCULATIONAHA.108.782458. Epub 2009 Apr 20.
Steve H Keller, Carmelle V Remillard, Olga Safrina, Ann Nicholson, Shenyuan L Zhang, Weihua Jiang, Nivruthi Vangala, Judd W Landsberg, Jian-Ying Wang, Patricia A Thistlethwaite, Richard N Channick, Ivan M Robbins, James E Loyd, Hossein A Ghofrani, Friedrich Grimminger, Ralph T Schermuly, Michael D Cahalan, Lewis J Rubin, Jason X-J Yuan
Affiliations
- PMID: 19380626
- PMCID: PMC2749566
- DOI: 10.1161/CIRCULATIONAHA.108.782458
A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension
Ying Yu et al. Circulation. 2009.
Abstract
Background: Excessive proliferation of pulmonary artery smooth muscle cells (PASMCs) plays an important role in the development of idiopathic pulmonary arterial hypertension (IPAH), whereas a rise in cytosolic Ca2+ concentration triggers PASMC contraction and stimulates PASMC proliferation. Recently, we demonstrated that upregulation of the TRPC6 channel contributes to proliferation of PASMCs isolated from IPAH patients. This study sought to identify single-nucleotide polymorphisms (SNPs) in the TRPC6 gene promoter that are associated with IPAH and have functional significance in regulating TRPC6 activity in PASMCs.
Methods and results: Genomic DNA was isolated from blood samples of 237 normal subjects and 268 IPAH patients. Three biallelic SNPs, -361 (A/T), -254(C/G), and -218 (C/T), were identified in the 2000-bp sequence upstream of the transcriptional start site of TRPC6. Although the allele frequencies of the -361 and -218 SNPs were not different between the groups, the allele frequency of the -254(C-->G) SNP in IPAH patients (12%) was significantly higher than in normal subjects (6%; P<0.01). Genotype data showed that the percentage of -254G/G homozygotes in IPAH patients was 2.85 times that of normal subjects. Moreover, the -254(C-->G) SNP creates a binding sequence for nuclear factor-kappaB. Functional analyses revealed that the -254(C-->G) SNP enhanced nuclear factor-kappaB-mediated promoter activity and stimulated TRPC6 expression in PASMCs. Inhibition of nuclear factor-kappaB activity attenuated TRPC6 expression and decreased agonist-activated Ca2+ influx in PASMCs of IPAH patients harboring the -254G allele.
Conclusions: These results suggest that the -254(C-->G) SNP may predispose individuals to an increased risk of IPAH by linking abnormal TRPC6 transcription to nuclear factor-kappaB, an inflammatory transcription factor.
Figures
Figure 1
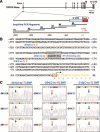
Identification of 3 SNPs in the 5′-regulatory region of the human TRPC6 gene. A, Schematic diagram of the TRPC6 gene and locations of the amplified PCR segments. B, The location of 3 SNPs, −361(A→T), −254(C→G), and −218(C→T), in the 5′-regulatory region of TRPC6. C, Representative sequence chromatographs for the 3 SNPs in IPAH patients. Arrow denotes position of the SNP. UTR indicates untranslated region.
Figure 2
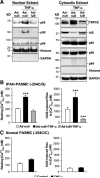
Nuclear NF-_κ_B proteins of PASMCs specifically bind to the −254(C→G) SNP–generated NF-_κ_B binding site. A, NF-_κ_B activation demonstrated by translocation of p50 and p65 from the cytoplasm to the nucleus. PASMCs from normal subjects were stimulated with (+) or without (−) TNF-α (10 ng/mL). B, Electrophoretic mobility shift assay and competition analysis. TNF-_α_–stimulated nuclear extracts from PASMCs were incubated with biotin-labeled double-stranded oligonucleotides corresponding to the 24-bp sequences containing the wild-type −254C or mutant −254G probes. Nuclear extract/oligonucleotide complexes (C1 and C2) are indicated by arrows. C, Antibody-mediated supershift assay. NF-_κ_B p50 and p65 antibodies identified complexes C1 as p50/p65 and C2 as p50/p50 NF-_κ_B–containing complexes.
Figure 3
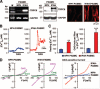
The basal and TNF-_α_–mediated promoter activities are enhanced in PASMCs transfected with the reporter constructs containing −254G allele. A, Schematic diagram (left) of the reporter constructs used for the promoter assay and the relative promoter activity in control and TNF-_α_–treated cells (right). The relative _β_-galactosidase activity (right) represents the mean±SE (plotted on a log scale) of 3 independent experiments performed in quadruplicate. *P<0.05, ***P<0.001 vs control cells. B, COS-7 cells transfected with _p_Blue(−335/+110) constructs that contain −254C or −254G allele. X-Gal staining was used to detect the _β_-galactosidase–stained cells. Empty (or promoterless) _p_Blue TOPO vector was used as control.
Figure 4
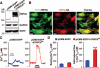
Upregulated TRPC6 expression in PASMCs from IPAH patients increases the resting [Ca2+]cyt and enhances agonist-mediated Ca2+ influx. A, mRNA and protein expression of TRPC6, determined by RT-PCR (left), Western blot (middle), and immunocyto-chemistry (right), respectively, is significantly higher in IPAH PASMCs than in control PASMCs from NPH patients. B, Representative records of [Ca2+]cyt changes in response to OAG (100 _μ_mol/L) in control (NPH) and IPAH PASMCs. C, Summarized data of the resting [Ca2+]cyt and the amplitude of the OAG-induced increase in [Ca2+]cyt in NPH and IPAH PASMCs. **P<0.01, ***P<0.001 vs NPH PASMCs. D, Representative currents recorded in NPH (left) and IPAH (middle) PASMCs before (Cont) and during (OAG) application with OAG (100 _μ_mol/L). OAG-sensitive currents, generated by subtracting the currents recorded during OAG from the control currents, in NPH and IPAH PASMCs are shown in the right panel.
Figure 5
Downregulation of TRPC6 with siRNA inhibits OAG-induced Ca2+ influx in PASMCs from IPAH patients. A, mRNA (left) and protein (right) expression levels of TRPC6, TRPC4, and GADPH are shown in IPAH PASMCs treated with scrambled siRNA, GADPH siRNA, or TRPC6 siRNA. B, Representative records of [Ca2+]cyt changes in response to OAG in IPAH PASMCs treated with scrambled siRNA (left) or TRPC6 siRNA (right). C, Summarized data of the resting [Ca2+]cyt (left) and OAG-induced [Ca2+]cyt increases (right) in IPAH PASMCs treated with scrambled or TRPC6 siRNA. **P<0.01, ***P<0.001 vs scrambled siRNA-treated control cells.
Figure 6
Overexpression of TRPC6 in normal PASMCs (with −254C/C genotype) enhances the OAG-induced increase in [Ca2+]cyt. A, Western blot analysis of TRPC6 in cells transfected with _p_CMS-EGFP or _p_CMS-EGFP-hTRPC6HA. B, Exogenous human TRPC6HA expression was identified by immunofluorescence staining. Normal PASMCs were transfected with _p_cDNA3-hTRPC6HA. HA epitope tag was stained by TRITC-conjugated anti-HA monoclonal antibody. TRPC6 antibody, followed by FITC-conjugated secondary antibody, was used to detect TRPC6 expression. C, Representative records of [Ca2+]cyt changes in response to OAG in PASMCs transfected with _p_CMS-EGFP or pcMS-EGFP-hTRPC6HA. D, Summarized data of the resting [Ca2+]cyt and the amplitude of the OAG-induced [Ca2+]cyt increases in cells transfected with _p_CMS-EGFP or _p_CMS-EGFP-hTRPC6HA. ***P<0.001 vs _p_CMS-EGFP cells.
Figure 7
Overexpression of the inhibitory subunit I_κ_B_α_ attenuates NF-κ_B–mediated TRPC6 expression and inhibits agonist-mediated Ca2+ influx in IPAH-PASMC. A, Overexpression of I_κ_B_α (Ad-I_κ_B) inhibits translocation of NF-_κ_B p50 and p65 into the nucleus (left) and diminishes TNF-α_–stimulated TRPC6 expression (right). GAPDH, histone (H2A), and p84 antibodies were used as controls for nuclear and cytoplasmic proteins. Experiments were reproduced 3 times. B, Summarized results showing that TNF-α increases the resting [Ca2+]cyt (left) and enhanced OAG-induced [Ca2+]cyt rise (right) in IPAH PASMCs (−254C/G), whereas I_κ_B_α (Ad-I_κ_B) abolishes the TNF-_α_–mediated enhancement of the resting [Ca2+]cyt and OAG-mediated Ca2+ influx. C, Summarized results showing that TNF-α negligibly affects the resting [Ca2+]cyt (left) and OAG-induced [Ca2+]cyt increase (right) in normal PASMCs (−254C/C).
Comment in
- Evidence for inflammatory signaling in idiopathic pulmonary artery hypertension: TRPC6 and nuclear factor-kappaB.
Hamid R, Newman JH. Hamid R, et al. Circulation. 2009 May 5;119(17):2297-8. doi: 10.1161/CIRCULATIONAHA.109.855197. Circulation. 2009. PMID: 19414653 No abstract available.
Similar articles
- Pathogenic role of calcium-sensing receptors in the development and progression of pulmonary hypertension.
Tang H, Yamamura A, Yamamura H, Song S, Fraidenburg DR, Chen J, Gu Y, Pohl NM, Zhou T, Jiménez-Pérez L, Ayon RJ, Desai AA, Goltzman D, Rischard F, Khalpey Z, Black SM, Garcia JG, Makino A, Yuan JX. Tang H, et al. Am J Physiol Lung Cell Mol Physiol. 2016 May 1;310(9):L846-59. doi: 10.1152/ajplung.00050.2016. Epub 2016 Mar 11. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26968768 Free PMC article. - Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry.
Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JX. Zhang S, et al. Am J Physiol Lung Cell Mol Physiol. 2007 May;292(5):L1202-10. doi: 10.1152/ajplung.00214.2006. Epub 2006 Dec 22. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17189322 - [Molecular Mechanism of Dihydropyridine Ca2+ Channel Blockers in Pulmonary Hypertension].
Yamamura A. Yamamura A. Yakugaku Zasshi. 2016;136(10):1373-1377. doi: 10.1248/yakushi.16-00086. Yakugaku Zasshi. 2016. PMID: 27725386 Review. Japanese. - Calcium-Sensing Receptor Regulates Cytosolic [Ca 2+ ] and Plays a Major Role in the Development of Pulmonary Hypertension.
Smith KA, Ayon RJ, Tang H, Makino A, Yuan JX. Smith KA, et al. Front Physiol. 2016 Nov 4;7:517. doi: 10.3389/fphys.2016.00517. eCollection 2016. Front Physiol. 2016. PMID: 27867361 Free PMC article. Review.
Cited by
- Cross talk between plasma membrane Na(+)/Ca (2+) exchanger-1 and TRPC/Orai-containing channels: key players in arterial hypertension.
Pulina MV, Zulian A, Baryshnikov SG, Linde CI, Karashima E, Hamlyn JM, Ferrari P, Blaustein MP, Golovina VA. Pulina MV, et al. Adv Exp Med Biol. 2013;961:365-74. doi: 10.1007/978-1-4614-4756-6_31. Adv Exp Med Biol. 2013. PMID: 23224895 Free PMC article. - Genetic Insights into Pulmonary Arterial Hypertension. Application of Whole-Exome Sequencing to the Study of Pathogenic Mechanisms.
Tang H, Desai AA, Yuan JX. Tang H, et al. Am J Respir Crit Care Med. 2016 Aug 15;194(4):393-7. doi: 10.1164/rccm.201603-0577ED. Am J Respir Crit Care Med. 2016. PMID: 27525458 Free PMC article. No abstract available. - Ion channels as therapeutic antibody targets.
Hutchings CJ, Colussi P, Clark TG. Hutchings CJ, et al. MAbs. 2019 Feb/Mar;11(2):265-296. doi: 10.1080/19420862.2018.1548232. Epub 2018 Dec 10. MAbs. 2019. PMID: 30526315 Free PMC article. Review. - Modulators of Transient Receptor Potential (TRP) Channels as Therapeutic Options in Lung Disease.
Dietrich A. Dietrich A. Pharmaceuticals (Basel). 2019 Feb 1;12(1):23. doi: 10.3390/ph12010023. Pharmaceuticals (Basel). 2019. PMID: 30717260 Free PMC article. Review. - TRPCs: Influential Mediators in Skeletal Muscle.
Choi JH, Jeong SY, Oh MR, Allen PD, Lee EH. Choi JH, et al. Cells. 2020 Apr 1;9(4):850. doi: 10.3390/cells9040850. Cells. 2020. PMID: 32244622 Free PMC article. Review.
References
- Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet. 2003;361:1533–1544. - PubMed
- Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. - PubMed
- Newman JH, Fanburg BL, Archer SL, Badesch DB, Barst RJ, Garcia JGN, Kao PN, Knowles JA, Loyd JE, McGoon MD, Morse JH, Nichols WC, Rabinovitch M, Rodman DM, Troy Stevens T, Tuder RM, Voelkel NF, Gail DB. Pulmonary arterial hypertension: future directions: report of a National Heart, Lung and Blood Institute/Office of Rare Diseases workshop. Circulation. 2004;109:2947–2952. - PubMed
- Robbins IM, Barst RJ, Rubin LJ, Gaine SP, Price PV, Morrow JD, Christman BW. Increased levels of prostaglandin D2 suggest macrophage activation in patients with primary pulmonary hypertension. Chest. 2001;120:1639–1644. - PubMed
- Yuan JX-J, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R29 HL054043/HL/NHLBI NIH HHS/United States
- R01 HL054043/HL/NHLBI NIH HHS/United States
- R37 NS014609/NS/NINDS NIH HHS/United States
- HL54043/HL/NHLBI NIH HHS/United States
- R01 HL066012/HL/NHLBI NIH HHS/United States
- R56 NS014609/NS/NINDS NIH HHS/United States
- HL64945/HL/NHLBI NIH HHS/United States
- NS14609/NS/NINDS NIH HHS/United States
- R01 HL064945/HL/NHLBI NIH HHS/United States
- R01 NS014609/NS/NINDS NIH HHS/United States
- HL66012/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous