Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12 - PubMed (original) (raw)
Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12
Stefania Violini et al. BMC Cell Biol. 2009.
Abstract
Background: Mesenchymal stem cells (MSCs) have been recently investigated for their potential use in regenerative medicine. MSCs, in particular, have great potential, as in various reports they have shown pluripotency for differentiating into many different cell types. However, the ability of MSCs to differentiate into tendon cells in vitro has not been fully investigated.
Results: In this study, we show that equine bone marrow mesenchymal stem cells (BM-MSCs), defined by their expression of markers such as Oct4, Sox-2 and Nanog, have the capability to differentiate in tenocytes. These differentiated cells express tendon-related markers including tenomodulin and decorin. Moreover we show that the same BM-MSCs can differentiate in osteocytes, as confirmed by alkaline phosphatase and von Kossa staining.
Conclusion: As MSCs represent an attractive tool for tendon tissue repair strategies, our data suggest that bone marrow should be considered the preferred MSC source for therapeutic approaches.
Figures
Figure 1
BM-derived MSCs express stem cell marker protein Oct4. Cells (5th passage) were fixed and incubated with antibodies directed against Oct4. Immunoreactivity was detected with avidin, biotinylated horseradish peroxydase and 3,3'-Diaminobenzidine (DAB) (1B). 1A: negative control, incubated only with secondary antibody. Magnification 20×.
Figure 2
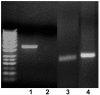
Gene expression analysis of BM-MSCs. The products from RT-PCR analysis of GAPDH (lane 1), Oct4 (lane 2), CD34 (lane 3), SOX-2 (lane 4) and Nanog (lane 5) mRNA expression at day 14 (passage 5) in BM-MSCs, showing the expression of mesenchymal stem cell-related markers and lack of expression of CD34.
Figure 3
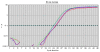
QRT-PCR of BM-MSCs for the Nanog, Oct4, Sox2 and GAPDH genes. Plot of QRT-PCR of Nanog (violet), Oct4 (green), Sox2 (blue) and GAPDH (red) on cDNA from BM-MSC cells. Cycle number is shown on the X-axis and emission intensity of a fluorescent reporter (Rn) is shown on the Y-axis. Fixed threshold is indicated as horizontal line.
Figure 4
Gene expression analysis of BM-MSC derived tenocytes. Products from RT-PCR analysis of tenomodulin (lane 1), P19 lipocalin (lane 3), GAPDH (lane 3) and decorin (lane 4) mRNA expression following 14 days stimulation of BM-MSC cells with BMP-12, resulting in differentiation into tenocytes.
Figure 5
Morphology of equine BM-MSC-derived tenocytes cultured in monolayer at day 20th. The cells at 5th passage in growth medium added with BMP-12 under phase contrast microscopy exhibited heterogeneous morphology with most cells fibroblast-like (A) and other elongated cells (B). Magnification 10×.
Figure 6
BM-MSCs osteogenic induction: alkaline phosphatase (AP) and von Kossa staining. After osteogenic induction cells showed a completely different morphology compared to untreated MSCs. (A, C): untreated control cells showing no staining for AP and von Kossa respectively (Magnification 10×). (B, D): AP and von Kossa positive staining in bone marrow-derived osteogenic cells. Magnification 20×.
Similar articles
- In Vitro Induction of Tendon-Specific Markers in Tendon Cells, Adipose- and Bone Marrow-Derived Stem Cells is Dependent on TGFβ3, BMP-12 and Ascorbic Acid Stimulation.
Perucca Orfei C, Viganò M, Pearson JR, Colombini A, De Luca P, Ragni E, Santos-Ruiz L, de Girolamo L. Perucca Orfei C, et al. Int J Mol Sci. 2019 Jan 3;20(1):149. doi: 10.3390/ijms20010149. Int J Mol Sci. 2019. PMID: 30609804 Free PMC article. - Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo.
Otabe K, Nakahara H, Hasegawa A, Matsukawa T, Ayabe F, Onizuka N, Inui M, Takada S, Ito Y, Sekiya I, Muneta T, Lotz M, Asahara H. Otabe K, et al. J Orthop Res. 2015 Jan;33(1):1-8. doi: 10.1002/jor.22750. Epub 2014 Oct 13. J Orthop Res. 2015. PMID: 25312837 Free PMC article. - BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo.
Lee JY, Zhou Z, Taub PJ, Ramcharan M, Li Y, Akinbiyi T, Maharam ER, Leong DJ, Laudier DM, Ruike T, Torina PJ, Zaidi M, Majeska RJ, Schaffler MB, Flatow EL, Sun HB. Lee JY, et al. PLoS One. 2011 Mar 11;6(3):e17531. doi: 10.1371/journal.pone.0017531. PLoS One. 2011. PMID: 21412429 Free PMC article. - Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies.
Costa-Almeida R, Calejo I, Gomes ME. Costa-Almeida R, et al. Int J Mol Sci. 2019 Jun 19;20(12):3002. doi: 10.3390/ijms20123002. Int J Mol Sci. 2019. PMID: 31248196 Free PMC article. Review. - Equine embryos and embryonic stem cells: defining reliable markers of pluripotency.
Paris DB, Stout TA. Paris DB, et al. Theriogenology. 2010 Sep 1;74(4):516-24. doi: 10.1016/j.theriogenology.2009.11.020. Epub 2010 Jan 13. Theriogenology. 2010. PMID: 20071015 Review.
Cited by
- Trends of regenerative tissue engineering for oral and maxillofacial reconstruction in veterinary medicine.
Purbantoro SD, Taephatthanasagon T, Purwaningrum M, Hirankanokchot T, Peralta S, Fiani N, Sawangmake C, Rattanapuchpong S. Purbantoro SD, et al. Front Vet Sci. 2024 Feb 21;11:1325559. doi: 10.3389/fvets.2024.1325559. eCollection 2024. Front Vet Sci. 2024. PMID: 38450027 Free PMC article. Review. - Mesoporous Particle Embedded Nanofibrous Scaffolds Sustain Biological Factors for Tendon Tissue Engineering.
Rinoldi C, Kijeńska-Gawrońska E, Heljak M, Jaroszewicz J, Kamiński A, Khademhosseini A, Tamayol A, Swieszkowski W. Rinoldi C, et al. ACS Mater Au. 2023 Jul 24;3(6):636-645. doi: 10.1021/acsmaterialsau.3c00012. eCollection 2023 Nov 8. ACS Mater Au. 2023. PMID: 38089667 Free PMC article. - Mesenchymal stem cells: An efficient cell therapy for tendon repair (Review).
Jiang L, Lu J, Chen Y, Lyu K, Long L, Wang X, Liu T, Li S. Jiang L, et al. Int J Mol Med. 2023 Aug;52(2):70. doi: 10.3892/ijmm.2023.5273. Epub 2023 Jun 30. Int J Mol Med. 2023. PMID: 37387410 Free PMC article. Review. - In Vitro and In Vivo Effects of IGF-1 Delivery Strategies on Tendon Healing: A Review.
Miescher I, Rieber J, Calcagni M, Buschmann J. Miescher I, et al. Int J Mol Sci. 2023 Jan 25;24(3):2370. doi: 10.3390/ijms24032370. Int J Mol Sci. 2023. PMID: 36768692 Free PMC article. Review. - Biological and Mechanical Factors and Epigenetic Regulation Involved in Tendon Healing.
Li ZJ, Yang QQ, Zhou YL. Li ZJ, et al. Stem Cells Int. 2023 Jan 9;2023:4387630. doi: 10.1155/2023/4387630. eCollection 2023. Stem Cells Int. 2023. PMID: 36655033 Free PMC article. Review.
References
- Stevens M. Stem cells in regenerative medicine. The Pharmaceutical Journal. 2005;275:695–698.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous