Progesterone receptor A-regulated gene expression in mammary organoid cultures - PubMed (original) (raw)
Progesterone receptor A-regulated gene expression in mammary organoid cultures
Sarah J Santos et al. J Steroid Biochem Mol Biol. 2009 Jul.
Abstract
Progesterone, through the progesterone receptor (PR), promotes development of the normal mammary gland and is implicated in the etiology of breast cancer. We identified PRA-regulated genes by microarray analysis of cultured epithelial organoids derived from pubertal and adult mouse mammary glands, developmental stages with differing progesterone responsiveness. Microarray analysis showed significant progestin (R5020)-regulation of 162 genes in pubertal organoids and 104 genes in adult organoids, with 68 genes regulated at both developmental stages. Greater induction of receptor activator of NFkappaB ligand and calcitonin expression was observed in adult organoids, suggesting possible roles in the differential progesterone responsiveness of the adult and pubertal mammary glands. Analysis of the R5020-responsive transcriptome revealed several enriched biological processes including cell adhesion, immune response, and survival. R5020 both induced Agtr1 and potentiated angiotensin II-stimulated proliferation, highlighting the functional significance of the latter process. Striking up-regulation of genes involved in innate immunity processes included the leukocyte chemoattractants serum amyloid A1, 2 and 3 (Saa1, 2, 3). In vivo analysis revealed that progesterone treatment increased SAA1 protein expression and leukocyte density in mammary gland regions undergoing epithelial expansion. These studies reveal novel targets of PRA in mammary epithelial cells and novel linkages of progesterone action during mammary gland development.
Figures
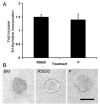
Fig. 1. Effect of R5020 and P on epithelial cell proliferation and organoid morphology
A. Mammary epithelial cells suspended in collagen I gels were cultured in BM, R5020 (20 nM), or P (20 nM). [3H] Thymidine incorporation into DNA was assayed after 3 d of culture. Each bar represents the mean ± SEM of triplicate values from a representative experiment. The data are expressed as the fold increase over the BM control. B. Organoid morphology was visualized in situ in collagen gels with the aid of an inverted microscope. Note the solid appearance of organoids from BM, whereas lumens are visible in R5020- and P-treated organoids. Scale bar = 100 μm.
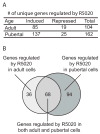
Fig. 2. R5020 treatment modulates levels of RNA expression in mouse mammary organoids
A. Table of the number of genes induced or repressed by R5020 in primary cultures from either adult or pubertal mice (fold change ≥ 2, P ≤ 0.05). B. Venn diagram of R5020-regulated genes in the adult and pubertal organoids.
Fig. 3. Fold inductions of twelve R5020-regulated genes as determined by quantitative RT-PCR
Three to four pubertal (●) and adult (○) primary cultures were analyzed for expression of each gene, and the average inductions are shown as solid black lines. * = P ≤ 0.05, Agtr1, Calca, Defb1 and Tnfsf11 were more highly induced by R5020 in adult organoids than pubertal organoids.
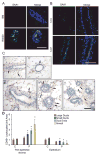
Fig. 4. Progestins induce SAA1 protein expression, and recruit leukocytes to the peri-epithelial stroma of small ducts, duct ends, and alveoli
A. Immunofluorescent detection of SAA1 (green) in adult organoids treated with basal media (BM) or R5020 for 24 hours. Nuclei (blue) were counterstained with DAPI. B. Immunofluorescent detection of SAA1 (green) in tissue sections from adult OVX mice treated with vehicle control (C) or progesterone (P) for 1 day. Nuclei (blue) were counterstained with DAPI. Scale bars (A,B) = 25 μm. C. Immunohistochemical detection of CD45-expressing leukocytes in mammary glands of OVX adult mice after 5 d treatment with vehicle control (i, ii) or progesterone (iii, iv, v). In control-treated sections, CD45+ leukocytes (brown cells marked by arrows) were sparsely localized in both ductal epithelium and peri-epithelial stroma (i, ii). In P-treated sections, CD45+ leukocytes were localized mainly in peri-epithelial stroma of ducts (iii), duct ends (iv), and alveoli (v). Scale bar = 50 μm. D. Quantitation of CD45+ leukocytes per unit area in the peri-epithelial stroma and epithelium of large ducts, small ducts, duct ends and alveoli after 5d C or P treatment. All structures were compared to control large ducts, and normalized to a value of 1 for CD45+ cells in control-treated large ducts. * = P ≤ 0.05.
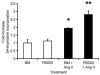
Fig. 5. Angiotensin II induces proliferation of organoids by itself and in synergy with R5020
Proliferation was measured by 3H-thymidine incorporation after 48-hour treatment with basal medium (BM), R5020 (20 nM), basal medium plus ANG II (BM + ANG II, 100nM), and R5020 plus ANG II (R5020 20 nM + ANG II 100 nM). Each bar represents the mean ± SEM of triplicate values from a representative experiment. * P≤0.05, BM + ANG II increased proliferation over BM alone. ** P≤0.05, R5020 + ANG II increased proliferation over any other treatment.
Similar articles
- Progestin-regulated luminal cell and myoepithelial cell-specific responses in mammary organoid culture.
Haslam SZ, Drolet A, Smith K, Tan M, Aupperlee M. Haslam SZ, et al. Endocrinology. 2008 May;149(5):2098-107. doi: 10.1210/en.2007-1398. Epub 2008 Jan 24. Endocrinology. 2008. PMID: 18218689 Free PMC article. - Extracellular matrix regulates ovarian hormone-dependent proliferation of mouse mammary epithelial cells.
Xie J, Haslam SZ. Xie J, et al. Endocrinology. 1997 Jun;138(6):2466-73. doi: 10.1210/endo.138.6.5211. Endocrinology. 1997. PMID: 9165037 - Progesterone receptor directly inhibits β-casein gene transcription in mammary epithelial cells through promoting promoter and enhancer repressive chromatin modifications.
Buser AC, Obr AE, Kabotyanski EB, Grimm SL, Rosen JM, Edwards DP. Buser AC, et al. Mol Endocrinol. 2011 Jun;25(6):955-68. doi: 10.1210/me.2011-0064. Epub 2011 Apr 28. Mol Endocrinol. 2011. PMID: 21527503 Free PMC article. - Progesterone regulation of stem and progenitor cells in normal and malignant breast.
Axlund SD, Sartorius CA. Axlund SD, et al. Mol Cell Endocrinol. 2012 Jun 24;357(1-2):71-9. doi: 10.1016/j.mce.2011.09.021. Epub 2011 Sep 16. Mol Cell Endocrinol. 2012. PMID: 21945473 Free PMC article. Review. - Interplay between progesterone and prolactin in mammary development and implications for breast cancer.
Lee HJ, Ormandy CJ. Lee HJ, et al. Mol Cell Endocrinol. 2012 Jun 24;357(1-2):101-7. doi: 10.1016/j.mce.2011.09.020. Epub 2011 Sep 16. Mol Cell Endocrinol. 2012. PMID: 21945475 Review.
Cited by
- Ovulation: Parallels With Inflammatory Processes.
Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Duffy DM, et al. Endocr Rev. 2019 Apr 1;40(2):369-416. doi: 10.1210/er.2018-00075. Endocr Rev. 2019. PMID: 30496379 Free PMC article. Review. - Progesterone receptor and Stat5 signaling cross talk through RANKL in mammary epithelial cells.
Obr AE, Grimm SL, Bishop KA, Pike JW, Lydon JP, Edwards DP. Obr AE, et al. Mol Endocrinol. 2013 Nov;27(11):1808-24. doi: 10.1210/me.2013-1077. Epub 2013 Sep 6. Mol Endocrinol. 2013. PMID: 24014651 Free PMC article. - Leukocytes in mammary development and cancer.
Coussens LM, Pollard JW. Coussens LM, et al. Cold Spring Harb Perspect Biol. 2011 Mar 1;3(3):a003285. doi: 10.1101/cshperspect.a003285. Cold Spring Harb Perspect Biol. 2011. PMID: 21123394 Free PMC article. Review. - C/EBPβ LIP and c-Jun synergize to regulate expression of the murine progesterone receptor.
Wang W, Do HN, Aupperlee MD, Durairaj S, Flynn EE, Miksicek RJ, Haslam SZ, Schwartz RC. Wang W, et al. Mol Cell Endocrinol. 2018 Dec 5;477:57-69. doi: 10.1016/j.mce.2018.06.001. Epub 2018 Jun 2. Mol Cell Endocrinol. 2018. PMID: 29870755 Free PMC article. - The role of ovarian sex steroids in metabolic homeostasis, obesity, and postmenopausal breast cancer: molecular mechanisms and therapeutic implications.
Boonyaratanakornkit V, Pateetin P. Boonyaratanakornkit V, et al. Biomed Res Int. 2015;2015:140196. doi: 10.1155/2015/140196. Epub 2015 Mar 19. Biomed Res Int. 2015. PMID: 25866757 Free PMC article. Review.
References
- Aupperlee M, Kariagina A, Osuch J, Haslam SZ. Progestins and breast cancer. Breast Dis. 2005;24:37–57. - PubMed
- Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72:163–172. - PubMed
- Lydon JP, Sivaraman L, Conneely OM. A reappraisal of progesterone action in the mammary gland. J Mammary Gland Biol Neoplasia. 2000;5:325–338. - PubMed
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. - PubMed
- Jacobsen BM, Richer JK, Sartorius CA, Horwitz KB. Expression profiling of human breast cancers and gene regulation by progesterone receptors. J Mammary Gland Biol Neoplasia. 2003;8:257–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous