Nanoparticle-mediated targeting of MAPK signaling predisposes tumor to chemotherapy - PubMed (original) (raw)
Nanoparticle-mediated targeting of MAPK signaling predisposes tumor to chemotherapy
Sudipta Basu et al. Proc Natl Acad Sci U S A. 2009.
Abstract
The MAPK signal transduction cascade is dysregulated in a majority of human tumors. Here we report that a nanoparticle-mediated targeting of this pathway can optimize cancer chemotherapy. We engineered nanoparticles from a unique hexadentate-polyD,L-lactic acid-co-glycolic acid polymer chemically conjugated to PD98059, a selective MAPK inhibitor. The nanoparticles are taken up by cancer cells through endocytosis and demonstrate sustained release of the active agent, resulting in the inhibition of phosphorylation of downstream extracellular signal regulated kinase. We demonstrate that nanoparticle-mediated targeting of MAPK inhibits the proliferation of melanoma and lung carcinoma cells and induces apoptosis in vitro. Administration of the PD98059-nanoparticles in melanoma-bearing mice inhibits tumor growth and enhances the antitumor efficacy of cisplatin chemotherapy. Our study shows the nanoparticle-mediated delivery of signal transduction inhibitors can emerge as a unique paradigm in cancer chemotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Development of PD98059 loaded nanoparticles. (A) Synthetic scheme for different PLGA-(PD98059)x conjugates. (B) Loading of PD98059 in mono (2)-, tri (7)- and hexa (9)- dentate PLGA expressed as microgram per milligram of polymer. (C) Representative transmission electron microscopy image of nanoparticles synthesized from hexadentate PLGA-(PD98059)6 conjugate. (Scale bar, 100 nm.)
Fig. 2.
Engineering pegylated nanoparticles. (A) Synthetic scheme for PEG-_b_-PLGA conjugate for engineering pegylated nanoparticles. Different ratio of PLGA-PEG: PLGA-[PD98059]6 results in nanoparticles of different size distribution, as measured by DLS (_y_-axis = intensity; _x_-axis = size in nm). (B) Graph shows physicochemical release kinetics of PD98059 when the nanoparticles are incubated with MDA-MB231, LLC, and B16/F10 cell lysates.
Fig. 3.
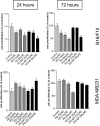
Effect of PD98059-loaded nanopaticles on cell proliferation. B16/F10 melanoma and MDA-MB-231 breast-cancer cells were incubated with free PD98059 (PD), PD98059-nanoparticle (PD-NP), or vehicle (control), for 24 or 72 h. MTS assays were used to determine the temporal cytotoxicity of increasing concentrations of free PD98059 and PD98059-nanoparticles (NP). Data are expressed as percent of vehicle-treated control (considered as 100%), and represents mean ± SEM from at least triplicates. *, P < 0.05 vs. vehicle control (ANOVA followed by Dunnet's post hoc test).
Fig. 4.
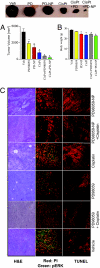
Combination therapy of PD98059-NP with cisplatin inhibits B16/F10 melanoma in xenograft mouse model. (A) Graph shows tumor volume of B16/F10 melanoma in different treatment groups, comparing the effects of PD98059-nanoparticle (PD-NP) + cisplatin, PD-NP, free PD98059, cisplatin, and free PD90859 + cisplatin. The control group received saline. Animals were treated with PD98059 (free or NP-conjugated) at a dose of 5 mg/kg through the tail vein. Cisplatin (1.25 mg/kg) was administered i.p. 1 day after the PD98059 dosing. Animals were killed on day 14. Each animal received 3 doses of the treatments. (B) Mean body weight of animals in different treatments as a measure of gross toxicity. All results are mean ± SEM (n = 6–8 per treatment group). *, P < 0.05 vs. vehicle (ANOVA followed by Newman Keul's post hoc test). (C) Representative images showing histological staining of the cross section of the excised tumors in different treatments at 20× magnifications. (Left) H&E staining of representative tumor cross-sections. (Middle) Tumor cross-section was immunostained for phospho-ERK (green), and counterstained with propidium iodide (red). (Right) Tumor sections were TUNEL-labeled for apoptosis with the use of Texas Red-labeled nucleotide. Images were captured using a Nikon Eclipse epifluorescence microscope.
Similar articles
- Salmonella enhance chemosensitivity in tumor through connexin 43 upregulation.
Chang WW, Lai CH, Chen MC, Liu CF, Kuan YD, Lin ST, Lee CH. Chang WW, et al. Int J Cancer. 2013 Oct 15;133(8):1926-35. doi: 10.1002/ijc.28155. Epub 2013 Apr 5. Int J Cancer. 2013. PMID: 23558669 - Development and characterization of hyaluronic acid modified PLGA based nanoparticles for improved efficacy of cisplatin in solid tumor.
Alam N, Koul M, Mintoo MJ, Khare V, Gupta R, Rawat N, Sharma PR, Singh SK, Mondhe DM, Gupta PN. Alam N, et al. Biomed Pharmacother. 2017 Nov;95:856-864. doi: 10.1016/j.biopha.2017.08.108. Epub 2017 Sep 10. Biomed Pharmacother. 2017. PMID: 28903181 - Cisplatin-loaded gelatin-poly(acrylic acid) nanoparticles: synthesis, antitumor efficiency in vivo and penetration in tumors.
Ding D, Zhu Z, Liu Q, Wang J, Hu Y, Jiang X, Liu B. Ding D, et al. Eur J Pharm Biopharm. 2011 Sep;79(1):142-9. doi: 10.1016/j.ejpb.2011.01.008. Epub 2011 Jan 25. Eur J Pharm Biopharm. 2011. PMID: 21272637 - PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect.
Acharya S, Sahoo SK. Acharya S, et al. Adv Drug Deliv Rev. 2011 Mar 18;63(3):170-83. doi: 10.1016/j.addr.2010.10.008. Epub 2010 Oct 20. Adv Drug Deliv Rev. 2011. PMID: 20965219 Review. - Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.
Pérez-Herrero E, Fernández-Medarde A. Pérez-Herrero E, et al. Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
Cited by
- An injectable microparticle formulation for the sustained release of the specific MEK inhibitor PD98059: in vitro evaluation and pharmacokinetics.
Naguib YW, Givens BE, Ho G, Yu Y, Wei SG, Weiss RM, Felder RB, Salem AK. Naguib YW, et al. Drug Deliv Transl Res. 2021 Feb;11(1):182-191. doi: 10.1007/s13346-020-00758-9. Drug Deliv Transl Res. 2021. PMID: 32378175 Free PMC article. - Drug delivery nanoparticles in skin cancers.
Dianzani C, Zara GP, Maina G, Pettazzoni P, Pizzimenti S, Rossi F, Gigliotti CL, Ciamporcero ES, Daga M, Barrera G. Dianzani C, et al. Biomed Res Int. 2014;2014:895986. doi: 10.1155/2014/895986. Epub 2014 Jul 2. Biomed Res Int. 2014. PMID: 25101298 Free PMC article. Review. - Engineered Nanoparticles Against MDR in Cancer: The State of the Art and its Prospective.
Ahmad J, Akhter S, Greig NH, Kamal MA, Midoux P, Pichon C. Ahmad J, et al. Curr Pharm Des. 2016;22(28):4360-4373. doi: 10.2174/1381612822666160617112111. Curr Pharm Des. 2016. PMID: 27319945 Free PMC article. Review. - Local, Sustained, and Targeted Co-Delivery of MEK Inhibitor and Doxorubicin Inhibits Tumor Progression in E-Cadherin-Positive Breast Cancer.
Kuhn PM, Russo GC, Crawford AJ, Venkatraman A, Yang N, Starich BA, Schneiderman Z, Wu PH, Vo T, Wirtz D, Kokkoli E. Kuhn PM, et al. Pharmaceutics. 2024 Jul 25;16(8):981. doi: 10.3390/pharmaceutics16080981. Pharmaceutics. 2024. PMID: 39204325 Free PMC article. - PLGA-loaded nanomedicines in melanoma treatment: Future prospect for efficient drug delivery.
Das S, Khuda-Bukhsh AR. Das S, et al. Indian J Med Res. 2016 Aug;144(2):181-193. doi: 10.4103/0971-5916.195024. Indian J Med Res. 2016. PMID: 27934796 Free PMC article. Review.
References
- Anonymous. Cancer Facts and Figures 2008. Atlanta: American Cancer Society 2008; 2008. American Cancer Society. www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf.
- Downward J. Targeting RAS signaling pathways in cancer chemotherapy. Nat Rev Cancer. 2003;3:11–22. - PubMed
- Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
- Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342–346. - PubMed
- Bos JL, et al. Prevalence of ras gene mutation in human colorectal cancers. Nature. 1987;327:293–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources