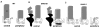Different roles for KIF17 and kinesin II in photoreceptor development and maintenance - PubMed (original) (raw)
Different roles for KIF17 and kinesin II in photoreceptor development and maintenance
Christine Insinna et al. Dev Dyn. 2009 Sep.
Abstract
Kinesin 2 family members are involved in transport along ciliary microtubules. In Caenorhabditis elegans channel cilia, kinesin II and OSM-3 cooperate along microtubule doublets of the axoneme middle segment, whereas OSM-3 alone works on microtubule singlets to elongate the distal segment. Among sensory cilia, vertebrate photoreceptors share a similar axonemal structure with C. elegans channel cilia, and deficiency in either kinesin II or KIF17, the homologue of OSM-3, results in disruption of photoreceptor organization. However, direct comparison of the two effects is confounded by the use of different species and knockdown strategies in prior studies. Here, we directly compare the effects of dominant-negative kinesin II and KIF17 expression in zebrafish cone photoreceptors. Our data indicate that dominant-negative kinesin II disrupts function at the level of the inner segment and synaptic terminal and results in cell death. In contrast, dominant-negative KIF17 has no obvious effect on inner segment or synaptic organization but has an immediate impact on outer segment assembly.
2009 Wiley-Liss, Inc.
Figures
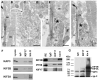
Fig. 1. KIF17 and kinesin II localization and co-immunoprecipitation
A-C. Immuno-EM localization of the KAP3 subunit of kinesin II in mouse rod photoreceptor cells. A. Longitudinal section through the connecting cilium and parts of the outer segment (OS). Bar = 400 nm. B. Cross section through the CC. Bar = 50 nm. C. Section through in the ER-Golgi region of the IS. Bars = 400 nm. Symbols (><) indicate position of adherens junctions at the outer limiting membrane. D-E. Immuno-EM localization of KIF17. D. Two slightly oblique sections through the CC. Bar = 50 nm in upper panel and 100 nm in lower panel. E. Two longitudinal images through the photoreceptor at the level of the CC. Arrow points to labeling in the axoneme of the OS. Asterisks indicate labeling in the collar-like extension of the apical IS. Bars = 400 nm. F. Co-immunoprecipitation from mouse retinal extracts of either kinesin II or KIF17 with IFT88. IP antibodies are shown at the top. Western blots were done with antibodies for KIF17, IFT88, and the three kinesin II subunits (KAP3, KIF3B and KIF3A); antibodies and are shown on the left. A mixture of IgGs was used as control for non-specific binding. G. IP experiment as in F from zebrafish retinal extract; the kinesin II antibody (K2.4) recognizes both KIF3A and KIF3B.
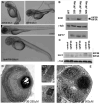
Fig. 2. Disruption of kif3B expression by an antisense morpholino oligonucleotide
A (upper panel). Images of three day old larvae that survived an injection of 500 μM (AKIF3B 500 μM) or 250 μM (AKIF3B 250 μM) of the translation-blocking morpholino. A (middle panel). A control injected embryo. A (lower panel). Three day old larva after injection with 250 μM of a splice-blocking morpholino (SpKIF3B). B. Western blots of whole embryo extracts showing the reduction of KIF3B and KIF3A in AKIF3B morphants at 3 dpf; anti-KIF17 and anti-γ-tubulin were used as controls. C. Western blot of three separate whole SpKif3B (250 μM) morphant and control embryo extracts showing similar depletion of KIF3B and KIF3A as observed in translation-blocking morphants (B above); anti- γ- tubulin was used as a loading control. D. Semi-thin section of the eye of an AKIF3B (250 μM) morphant at 3 dpf. Bar = 10 μm. Insets: EM views of AKIF3B (250 μM) photoreceptors. Bar = 2.5 μm. E. Semi-thin section of the eye of an AKIF3B (500 μM) morphant at 3 dpf. Note the absence of retinal lamination and photoreceptor differentiation. Bar = 10 μm.
Fig. 3. Localization of Ta-CP directed expression of GFP, DNKIF3B and DNKIF17 and cone opsin localization at 5 dpf
The Ta-CP promoter up stream of GFP, DNKIF3B or DNKIF17 was injected at the 1 cell stage. A. In Ta-CP/GFP embryos GFP fluorescence was seen throughout the outer retina and cone opsin was highly localized to the OS. Bar = 10 μm and applies to panels A-C. B. In Ta-CP/DNKIF3B embryos transgene expression (GFP, green) was similar to controls, but cone opsin was mislocalized to the perinuclear region and outer plexiform layer (arrow) across the entire retina. Inset: Higher power image showing cone opsin mislocalization to the synaptic layer (arrow). Bar = 10 μm. C. In Ta-CP/DNKIF17 embryos transgene expression (GFP, green) was distributed across the entire retina, but was largely restricted to the IS (see inset). Cone opsin was in the OS and there was no evidence of mislocalization. Inset: Higher power image showing lack of mislocalization of cone opsin and strong accumulation of DNKIF17 in the IS. Bar = 10 μm.
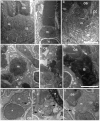
Fig. 4. Light microscopy of retinae of control, DNKIF3B and DNKIF17 embryos at 5 dpf
A-B. Low power images of semi-thin plastic sections of DNKIF3B (A) and DNKIF17 (B) eyes. Central (center) and peripheral (periph) retinal regions are labeled. Bar in B = 10 μm. C. Higher power image of the outer retina of a control eye; the periphery is on the right (Periph). Cone and rod OS are indicated by arrows. D-E. Higher power images of outer retina of DNKIF3B (D) and DNKIF17 (E) eyes; the periphery is on the right (periph). White arrows in D indicate condensed nuclei and cell bodies of dying cones. White arrows in E indicate cones with very short or missing OS. A normal rod OS is indicated in E, The bar in E = 10 μm and applies to C-E).
Fig. 5. Summary of the structural phenotypes observed in DNKIF3B and DNKIF17 embryos
A. In wild type retinae, cones of the central retina are more developed with longer OS than those at the periphery. CC, connecting cilium. CP, calycal process. B. In embryos expressing DNKIF3B many cells in the central and peripheral retina exhibit a condensed morphology suggesting an apoptotic state. In the periphery many uncondensed cones also exhibit IS vesicles and accumulation of dense material while retaining normal OS structure. C. In embryos expressing DNKIF17 cones in the central retina with normal IS exhibit accumulations of vesicles in the proximal OS opposite the cilium, but this phenotype is not seen in all cells. In the periphery, OS do not elongate and are often vesiculated. Cones do not exhibit features associated with excess apoptosis.
Fig. 6. Accumulation of large vacuoles and dense material in the IS of cones expressing DNKIF3B
A. EM view of cones at 5 days after injection of the Ta-CP driving GFP alone (control). Note the normal structure of OS discs and IS with accumulation of mitochondria (m) in the ellipsoid region. B and C. EM view of cones at 5 days after injection of the Ta-CP driving DNKIF3B showing mild IS defects. This was primarily enlargement of the cytoplasmic area around the mitochondria. Arrow indicates large vesicle below the CC. n, photoreceptor nucleus. g, golgi apparatus. Bar in C = 1.5 μm. D. EM view of a cross-section through the IS of a cone with a mild IS phenotype involving enlargement of the cytoplasmic area around mitochondria; er, endoplasmic reticulum. The upper arrow indicates accumulation of large vesicles in the ellipsoid region. The lower arrow indicates a Golgi region. E. EM view of a cone in the central retina that has a highly condensed IS between the nucleus (n) and mitochondrial rich (m) ellipsoid region. OS discs are normal. F. Cone with a highly condensed nucleus and IS with normal OS organization. Bar = 2.5 μm. G-H. Examples of cones accumulating vesicles and large vacuoles (arrows) within the IS surrounding the mitochondria. Asterisk in H indicates condensed nucleus of an adjacent cone.
Fig. 7. Lack of synaptic ribbons in cone pedicles of DNKIF3B expressing embryos
A-C. Control pedicles showing post-synaptic invaginations (large arrows) and synaptic ribbons (small arrows) associated with pre-synaptic membranes. D-F. DNKIF17 cone pedicles showing post-synaptic invaginations (large arrows) and synaptic ribbons (small arrows) associated with pre-synaptic membranes. G-I. DNKIF3B cone pedicles showing post-synaptic invaginations (large arrows) without synaptic ribbons. Occasionally, ribbons are seen dissociated from presynaptic membranes (small arrow in I). Condensed nuclei and pedicles (asterisks) of dying cells are seen in G. Insets in C, F, and I are enlargements of invaginations to show association of ribbons with the presynaptic membrane. Bars in I and in inset = 500 nm.
Fig. 8. Disruption of the OS structure in cones expressing DNKIF17
A-D. EM views of cones at the retinal periphery at 5 days after injection of Ta-CP driving DNKIF17. Note disrupted OS structure with failure to complete disc edges as shown by arrows. E. EM view of a cone in the central retina accumulating vesicular membranes within the base of the OS (arrows) opposite the cilium (CC). F. Normal rod photoreceptor. All bars = 2.5 μm; the bar in C applies to A-C.
Similar articles
- Kinesin-2 family motors in the unusual photoreceptor cilium.
Malicki J, Besharse JC. Malicki J, et al. Vision Res. 2012 Dec 15;75:33-6. doi: 10.1016/j.visres.2012.10.008. Epub 2012 Oct 31. Vision Res. 2012. PMID: 23123805 Free PMC article. Review. - The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development.
Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. Insinna C, et al. Dev Biol. 2008 Apr 1;316(1):160-70. doi: 10.1016/j.ydbio.2008.01.025. Epub 2008 Jan 31. Dev Biol. 2008. PMID: 18304522 Free PMC article. - Kif17 phosphorylation regulates photoreceptor outer segment turnover.
Lewis TR, Kundinger SR, Link BA, Insinna C, Besharse JC. Lewis TR, et al. BMC Cell Biol. 2018 Nov 20;19(1):25. doi: 10.1186/s12860-018-0177-9. BMC Cell Biol. 2018. PMID: 30458707 Free PMC article. - Analysis of KIF17 distal tip trafficking in zebrafish cone photoreceptors.
Bader JR, Kusik BW, Besharse JC. Bader JR, et al. Vision Res. 2012 Dec 15;75:37-43. doi: 10.1016/j.visres.2012.10.009. Epub 2012 Oct 23. Vision Res. 2012. PMID: 23099049 Free PMC article. - Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia.
Silverman MA, Leroux MR. Silverman MA, et al. Trends Cell Biol. 2009 Jul;19(7):306-16. doi: 10.1016/j.tcb.2009.04.002. Epub 2009 Jun 25. Trends Cell Biol. 2009. PMID: 19560357 Review.
Cited by
- The cytoplasmic tail of rhodopsin triggers rapid rod degeneration in kinesin-2 mutants.
Feng D, Chen Z, Yang K, Miao S, Xu B, Kang Y, Xie H, Zhao C. Feng D, et al. J Biol Chem. 2017 Oct 20;292(42):17375-17386. doi: 10.1074/jbc.M117.784017. Epub 2017 Aug 30. J Biol Chem. 2017. PMID: 28855254 Free PMC article. - Kinesin-2 family motors in the unusual photoreceptor cilium.
Malicki J, Besharse JC. Malicki J, et al. Vision Res. 2012 Dec 15;75:33-6. doi: 10.1016/j.visres.2012.10.008. Epub 2012 Oct 31. Vision Res. 2012. PMID: 23123805 Free PMC article. Review. - Functional compartmentalization of photoreceptor neurons.
Malhotra H, Barnes CL, Calvert PD. Malhotra H, et al. Pflugers Arch. 2021 Sep;473(9):1493-1516. doi: 10.1007/s00424-021-02558-7. Epub 2021 Apr 20. Pflugers Arch. 2021. PMID: 33880652 Free PMC article. Review. - Direct regulation of microtubule dynamics by KIF17 motor and tail domains.
Acharya BR, Espenel C, Kreitzer G. Acharya BR, et al. J Biol Chem. 2013 Nov 8;288(45):32302-32313. doi: 10.1074/jbc.M113.494989. Epub 2013 Sep 26. J Biol Chem. 2013. PMID: 24072717 Free PMC article. - A role for the primary cilium in Notch signaling and epidermal differentiation during skin development.
Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. Ezratty EJ, et al. Cell. 2011 Jun 24;145(7):1129-41. doi: 10.1016/j.cell.2011.05.030. Cell. 2011. PMID: 21703454 Free PMC article.
References
- Baker SA, Freeman K, Luby-Phelps K, Pazour GJ, Besharse JC. IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. J Biol Chem. 2003;278:34211–34218. - PubMed
- Beech PL, Pagh-Roehl K, Noda Y, Hirokawa N, Burnside B, Rosenbaum JL. Localization of kinesin superfamily proteins to the connecting cilium of fish photoreceptors. J Cell Sci. 1996;109(Pt 4):889–897. - PubMed
- Branchek T, Bremiller R. The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J Comp Neurol. 1984;224:107–115. - PubMed
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. - PubMed
- Chu PJ, Rivera JF, Arnold DB. A role for Kif17 in transport of Kv4.2. J Biol Chem. 2006;281:365–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 EY014537-06A1/EY/NEI NIH HHS/United States
- T32 EY014537-05/EY/NEI NIH HHS/United States
- R01 EY003222-28A2/EY/NEI NIH HHS/United States
- P30 EY001931-31/EY/NEI NIH HHS/United States
- R01 EY002414-33/EY/NEI NIH HHS/United States
- R01 EY003222-27/EY/NEI NIH HHS/United States
- R01 EY002414-33S1/EY/NEI NIH HHS/United States
- EY03222/EY/NEI NIH HHS/United States
- P30 EY001931/EY/NEI NIH HHS/United States
- R01 EY003222/EY/NEI NIH HHS/United States
- T32-EY014537/EY/NEI NIH HHS/United States
- S10 RR015679-01/RR/NCRR NIH HHS/United States
- P30 EY001931-30/EY/NEI NIH HHS/United States
- T32 EY014537/EY/NEI NIH HHS/United States
- R01 EY002414/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases