Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease - PubMed (original) (raw)
Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease
Rodrigo A Quintanilla et al. J Biol Chem. 2009.
Abstract
In Alzheimer disease (AD) mitochondrial abnormalities occur early in the pathogenic process and likely play a significant role in disease progression. Tau is a microtubule-associated protein that is abnormally processed in AD, and a connection between tau pathology and mitochondrial impairment has been proposed. However, few studies have examined the relationship between pathological forms of tau and mitochondrial dysfunction. We recently demonstrated that inducible expression of tau truncated at Asp-421 to mimic caspase cleavage (T4C3) was toxic to immortalized cortical neurons compared with a full-length tau isoform (T4). In this study we investigated the effects of T4C3 on mitochondrial function. Expression of T4C3 induced mitochondrial fragmentation and elevated oxidative stress levels in comparison with T4-expressing cells. Thapsigargin treatment of T4 or T4C3 cells, which causes an increase in intracellular calcium levels, resulted in a significant decrease in mitochondrial potential and loss of mitochondrial membrane integrity in T4C3 cells when compared with cells expressing T4. The mitochondrial fragmentation and mitochondrial membrane damage were ameliorated in T4C3 cells by pretreatment with cyclosporine A or FK506, implicating the calcium-dependent phosphatase calcineurin in these pathogenic events. Increased calcineurin activity has been reported in AD brain, and thus, inhibition of this phosphatase may provide a therapeutic target for the treatment of AD.
Figures
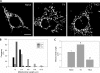
FIGURE 1.
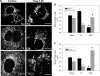
Expression of T4C3 induces mitochondrial fragmentation in immortalized cortical neurons. Mitochondria in naïve, T4, and T4C3 cells were labeled with the mitochondria-specific marker MTG. Mitochondria in cells expressing T4 presented with the expected tubular, rod-like morphology, whereas cells expressing T4C3 exhibited more rounded and fragmented mitochondria. A, representative confocal images of cells labeled with MTG. B, more than 90% of mitochondria in cells expressing T4C3 were less than 2 μm in length, whereas the majority of mitochondria in naïve and T4 cells ranged between 2 and 6 μm. C, quantitation revealed that mitochondria in T4C3 cells showed more than a 2-fold decrease in average mitochondrial length as compared with mitochondria in cells expressing T4. Data are the mean ± S.E. (bars). *, p < 0.01 compared with T4C3 untreated cells; **, p < 0.05 compared with untreated naïve cells. p < 0.01 (*) and p < 0.05 (**) were by unpaired Student's t test. The bar scale represents 10 μm.
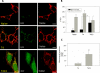
FIGURE 2.
Caspase-cleaved tau expression increased mitochondrial ROS production in cortical neurons. Naïve, T4, and T4C3 cells were loaded with 2.7-DCF and TMRM for 40 min to measure changes in mitochondrial ROS production using confocal microscopy in situ. A, representative confocal images from immortalized cortical neurons loaded with 2.7-DCF/TMRM in basal conditions. Caspase-cleaved tau (T4C3) expression increased mitochondrial ROS production in immortalized cortical neurons. B, quantified mitochondrial potential levels and ROS production data from three independent experiments reveal that mitochondrial ROS levels in T4C3 cells are significantly elevated in comparison with naïve and T4 cells. Mitochondrial ROS levels were estimated by analyzing the areas of co-localization between 2.7-DCF/TMRM signals. Data are the mean ± S.E. (bars). *, p < 0.05 T4C3 cells compared with T4 cells. C, to corroborate the finding presented above, we evaluated mitochondrial superoxide levels in cortical neurons using MitoSOX. Untreated T4C3 cells showed significantly increased mitochondrial superoxide levels in comparison with T4 cells. Data are the mean ± S.E. (bars), and values are from three separate experiments. *, p < 0.05 T4C3 cells compared with T4 cells. The bar scale represents 10 μm.
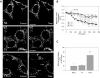
FIGURE 3.
Expression of T4C3 induces mitochondrial dysfunction in cortical neurons treated with thapsigargin. Mitochondria in cells expressing T4C3 respond differently to increases in intracellular calcium levels in acute experiments. A, Fluo-3 was used to measure cytosolic calcium levels in the cells after thapsigargin (Thap, 1 μ
m
) treatment for 30 min. We found no significant difference in cytosolic calcium increases between cells expressing T4 or T4C3 after treatment. B, mitochondrial calcium levels were measured after treatment using Rhod-2. Mitochondrial calcium levels increased in cells expressing T4, whereas mitochondria in cells expressing T4C3 showed a decrease in calcium levels. C, mitochondrial membrane potential was measured using TMRM. TMRM fluorescence levels decreased significantly in T4C3 cells but not in naïve and T4 cells after thapsigargin treatment. D, the graph represents quantification of mitochondrial potential fluorescence intensities as relative units, which shows that T4C3 cells treated with thapsigargin for 30 min exhibit a pronounced lost of mitochondrial potential. Data are the mean ± S.E. (bars) from three separate experiments. *, p < 0.05, T4C3 cells compared with T4 cells.
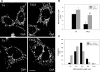
FIGURE 4.
Caspase-cleaved tau expression induces mitochondrial damage in immortalized cortical neurons. Naïve (Na), T4, and T4C3 cells were loaded with MTG to evaluate mitochondrial membrane integrity damage (see
supplemental
Fig. 1) during 30 min of thapsigargin (Thap) treatment. A, representative confocal images from untreated and thapsigargin-treated immortalized cortical neurons that indicate calcium overload significantly induces mitochondrial damage in T4C3 cells (see the white arrows). B, representative trends from three independent experiments of mitochondrial integrity damage induced by thapsigargin in naïve, T4, and T4C3 cells that show a significant decrease in MTG fluorescence levels in T4C3 cells, in comparison with naïve and T4 cells. C, graph representing quantification of mitochondrial integrity damage (thapsigargin-treated cells), expressed as percentage of mitochondrial damage, which shows that T4C3 cells treated with thapsigargin exhibit a pronounced lost of mitochondrial membrane integrity. Data are the mean ± S.E. (bars) from three separate experiments. *, p < 0.05 T4C3 cells compared with T4 cells. The bar scale represents 10 μm.
FIGURE 5.
Pretreatment with CsA attenuates mitochondrial fragmentation in T4C3 cells. T4 and T4C3 cells pretreated with 1 μ
m
CsA for 2 h were loaded with MTG to evaluate mitochondrial morphology changes. A, representative confocal images of cortical neurons that indicate pretreatment with CsA greatly diminishes the mitochondrial fragmentation observed in T4C3 cells (see Fig. 1 for comparison). Na, naïve; Thap, thapsigargin. B, quantification of three independent experiments revealed that the average length of mitochondria in both cell types increased in response to CsA pretreatment, although the effect was not as robust in cells expressing T4. Data are the mean ± S.E. (bars). *, p < 0.01 compared with T4 untreated cells; **, p < 0.05 compared with untreated T4C3 cells. p < 0.01 (*) and p < 0.05 (**) were by unpaired Student's t test. C, CsA treatment increased average mitochondrial length in T4C3 cells with more than 70% of mitochondria being more than 2 μm in length (for comparison see Fig. 1_B_), whereas the majority of mitochondria in T4 cells ranged between 2 and 6 μm, as was observed in control conditions (for comparison see Fig. 1_B_). The bar scale represents 10 μm.
FIGURE 6.
CsA prevents mitochondrial damage and depolarization in response to thapsigargin in caspase-cleaved tau-expressing cortical neurons. A, T4 and T4C3 cells were pretreated with 1 μ
m
CsA for 2 h and loaded with MTG to evaluate the extent of mitochondrial damage induced by thapsigargin treatment. The graph shows representative trends from three independent experiments in T4 and T4C3 cells that reveal a significant retention of MTG fluorescence in T4C3 cells after thapsigargin treatment, in comparison with T4 cells (for comparison, see Fig. 4). B, graph represents quantification of mitochondrial integrity damage (thapsigargin-treated cells (black bars); CsA + thapsigargin treated cells (gray bars)) expressed as percentage mitochondrial damage, which shows that T4C3 cells treated with CsA are protected against the loss of mitochondrial membrane integrity induced by 30 min of treatment with thapsigargin. Data are the mean ± S.E. (bars) from three separate experiments. **, p < 0.05 compared with T4 cells treated with thapsigargin; ##, p < 0.05 was compared with T4C3 cells treated with thapsigargin. C, T4 and T4C3 cells were pretreated with 1 μ
m
CsA for 2 h, and mitochondrial membrane potential was determined using TMRM. Pretreatment with CsA was able to ameliorate mitochondrial potential loss induced by thapsigargin in T4C3 cells. The graph represents quantification of TMRM fluorescence intensities as relative units, which shows that T4C3 cells treated with CsA plus thapsigargin exhibit a significant increase in the relative mitochondrial potential levels. D, data are the mean ± S.E. (bars) from three separate experiments. *, p < 0.05 compared with T4 cells treated with thapsigargin; **, p < 0.01 compared with T4C3 cells treated with thapsigargin.
FIGURE 7.
FK506 prevents mitochondrial fragmentation in caspase-cleaved tau-expressing cells. T4 and T4C3 cells were pretreated with 0.6 μ
m
FK506 for 2 h and then loaded with MTG to evaluate mitochondrial morphology changes. A, representative fluorescence images of cortical cells that indicate pretreatment with FK506 significantly diminishes the mitochondrial fragmentation observed in T4C3 cells (see Fig. 1 for comparison). B, quantification of three independent experiments revealed that the average length of mitochondria in T4C3 cells increased significantly in response to FK506 pretreatment. Data are the mean ± S.E. (bars). *, p < 0.01 compared with T4 untreated cells; **, p < 0.05 compared with untreated T4C3 cells. p < 0.01 (*) and p < 0.05 (**) by unpaired Student's t test. C, CsA and FK506 treatment increased average mitochondrial length in T4C3 cells with more than 70% of mitochondria more than 2 μm in length (for comparison sees Fig. 1_B_). Naïve cells presented an average mitochondrial length of 2.5 μm, and CsA and FK506 values were expressed as a function of this mitochondrial length. Data are the mean ± S.E. (bars). *, p < 0.01 compared with T4 untreated cells. The bar scale represents 10 μm.
FIGURE 8.
Inhibition of calcineurin prevents mitochondrial depolarization induced by thapsigargin in T4C3 cells. A, T4 and T4C3 cells were pretreated with 0.6 μ
m
FK506 for 2 h, and mitochondrial membrane potential was determined using TMRM. Pretreatment with FK506 was able to prevent mitochondrial potential loss induced by thapsigargin in T4C3 cells. The graph trends represent quantification of TMRM fluorescence intensities as relative units, which shows that T4C3 cells treated with FK506 plus thapsigargin exhibit a significant increase in the relative mitochondrial potential levels. B, data are the mean ± S.E. (bars) from three separate experiments. *, p < 0.05 compared with T4 cells treated with thapsigargin; **, p < 0.01 compared with T4C3 cells treated with thapsigargin.
FIGURE 9.
Effects of chronic treatment with thapsigargin in mitochondrial morphology in immortalized cortical neurons. Naïve, T4, and T4C3 cells were treated with 1 μ
m
thapsigargin for 6 h before analysis of mitochondrial morphology. Mitochondria in naïve, T4, and T4C3 cells were labeled with the mitochondria-specific marker MTG. Mitochondria in cells expressing T4 showed significant mitochondrial fragmentation after thapsigargin treatment, whereas cells expressing T4C3 exhibited an evident mitochondrial swelling morphology after treatment. Interestingly, naïve cells presented a similar mitochondrial morphology pattern that untreated naïve cells. A, representative confocal images. B, quantitation revealed that mitochondria in T4C3 cells showed more than a 2-fold increase in average mitochondrial length as compared with mitochondria in cells expressing T4 treated with thapsigargin. C, quantitation of three independent experiments suggests that T4 cells significantly reduce the mitochondrial area after thapsigargin treatment; however, T4C3 cells show a greatly increase mitochondrial area after exposure to thapsigargin for 6 h, an effect that is due to mitochondrial swelling. Data are the mean ± S.E. (bars). *, p < 0.01 compared with T4 untreated cells; **, p < 0.05 compared with untreated T4C3 cells; #, p < 0.05 compared with untreated T4 cells. p < 0.01 (*) and p < 0.05 (**), unpaired Student's t test. The bar scale represents 10 μm.
Similar articles
- Caspase-Cleaved Tau Impairs Mitochondrial Dynamics in Alzheimer's Disease.
Pérez MJ, Vergara-Pulgar K, Jara C, Cabezas-Opazo F, Quintanilla RA. Pérez MJ, et al. Mol Neurobiol. 2018 Feb;55(2):1004-1018. doi: 10.1007/s12035-017-0385-x. Epub 2017 Jan 13. Mol Neurobiol. 2018. PMID: 28084594 - Immortalized cortical neurons expressing caspase-cleaved tau are sensitized to endoplasmic reticulum stress induced cell death.
Matthews-Roberson TA, Quintanilla RA, Ding H, Johnson GV. Matthews-Roberson TA, et al. Brain Res. 2008 Oct 9;1234:206-12. doi: 10.1016/j.brainres.2008.07.111. Epub 2008 Aug 7. Brain Res. 2008. PMID: 18718455 Free PMC article. - Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation.
Ding H, Matthews TA, Johnson GV. Ding H, et al. J Biol Chem. 2006 Jul 14;281(28):19107-14. doi: 10.1074/jbc.M511697200. Epub 2006 May 10. J Biol Chem. 2006. PMID: 16687396 - The Role of Mitochondrial Impairment in Alzheimer´s Disease Neurodegeneration: The Tau Connection.
Quntanilla RA, Tapia-Monsalves C. Quntanilla RA, et al. Curr Neuropharmacol. 2020;18(11):1076-1091. doi: 10.2174/1570159X18666200525020259. Curr Neuropharmacol. 2020. PMID: 32448104 Free PMC article. Review. - Pathological Impact of Tau Proteolytical Process on Neuronal and Mitochondrial Function: a Crucial Role in Alzheimer's Disease.
Olesen MA, Quintanilla RA. Olesen MA, et al. Mol Neurobiol. 2023 Oct;60(10):5691-5707. doi: 10.1007/s12035-023-03434-4. Epub 2023 Jun 19. Mol Neurobiol. 2023. PMID: 37332018 Review.
Cited by
- A caspase cleaved form of tau is preferentially degraded through the autophagy pathway.
Dolan PJ, Johnson GV. Dolan PJ, et al. J Biol Chem. 2010 Jul 16;285(29):21978-87. doi: 10.1074/jbc.M110.110940. Epub 2010 May 13. J Biol Chem. 2010. PMID: 20466727 Free PMC article. - Cogs in the autophagic machine-equipped to combat dementia-prone neurodegenerative diseases.
de Wet S, Theart R, Loos B. de Wet S, et al. Front Mol Neurosci. 2023 Aug 31;16:1225227. doi: 10.3389/fnmol.2023.1225227. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37720551 Free PMC article. Review. - Are tangles as toxic as they look?
Spires-Jones TL, Kopeikina KJ, Koffie RM, de Calignon A, Hyman BT. Spires-Jones TL, et al. J Mol Neurosci. 2011 Nov;45(3):438-44. doi: 10.1007/s12031-011-9566-7. Epub 2011 Jun 3. J Mol Neurosci. 2011. PMID: 21638071 Free PMC article. Review. - Tau Proteolysis in the Pathogenesis of Tauopathies: Neurotoxic Fragments and Novel Biomarkers.
Quinn JP, Corbett NJ, Kellett KAB, Hooper NM. Quinn JP, et al. J Alzheimers Dis. 2018;63(1):13-33. doi: 10.3233/JAD-170959. J Alzheimers Dis. 2018. PMID: 29630551 Free PMC article. Review. - Selective Neuron Vulnerability in Common and Rare Diseases-Mitochondria in the Focus.
Paß T, Wiesner RJ, Pla-Martín D. Paß T, et al. Front Mol Biosci. 2021 Jun 30;8:676187. doi: 10.3389/fmolb.2021.676187. eCollection 2021. Front Mol Biosci. 2021. PMID: 34295920 Free PMC article. Review.
References
- García-Sierra F., Mondragón-Rodríguez S., Basurto-Islas G. (2008) J. Alzheimers Dis. 14, 401–409 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical