Estrogen regulation of thrombospondin-1 in human breast cancer cells - PubMed (original) (raw)
Estrogen regulation of thrombospondin-1 in human breast cancer cells
Salman M Hyder et al. Int J Cancer. 2009.
Abstract
Expression of thrombospondin-1 (TSP-1), a large extracellular matrix protein, has been associated with modulation of angiogenesis and tumor growth. Both pro and antiangiogenic properties of TSP-1 have been described, and the role of TSP-1 expression in the growth and progression of human breast cancer is not clear. Because estrogens cause progression of many breast cancers, and estradiol (E2) downregulates a TSP-1 receptor, we examined whether TSP-1 is regulated by estrogen and involved in tumor progression. E2 induced TSP-1 expression in T47-D and MCF-7 breast cancer cells in vitro within 3 to 6 hr; the induction was blocked by the anti-estrogen ICI 182,780, indicating that estrogen receptors (ER) are necessary for this effect. Furthermore, E2 caused the production of TSP-1 protein from tumor cells in an ER-alpha-dependent manner. The E2-mediated TSP-1 RNA induction was dose-dependent and blocked by actinomycin D, indicating that the response to E2 was at least partly transcriptional. Transfection studies with deletion constructs of the TSP-1 promoter identified an estrogen-responsive region in the human TSP-1 promoter, located between -2,200 and -1,792 bp upstream of the transcription start site. An antibody against TSP-1 restricted the proliferation of E2-dependent MCF-7 cells in vitro and in vivo. A panel of breast cancer cells proliferated in the presence of low concentrations of exogenous TSP-1, whereas higher concentrations inhibited proliferation. A real-time PCR analysis showed that E2 also induced TSP-1 mRNA in the normal mammary glands of immature ovariectomized mice in an ER-dependent manner. In summary, we report the novel observation that TSP-1 production is directly controlled by estrogens in ER-positive breast cancer cells, and the released protein has pro-growth regulatory functions. Consequently, we propose that TSP-1 could be a therapeutic target for anti-tumor therapy in early-stage tumors. (c) 2009 UICC.
Figures
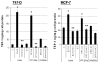
Figure 1. E2 induces TSP-1 message and protein in breast cancer cells
T47-D and MCF-7 cells were cultured and total RNA prepared and assessed for the expression of TSP-1 mRNA by Northern blot analysis. The blots were then stripped and probed for 28S RNA as a loading control. (A) T47-D cells were treated with 10 nM E2 for 1, 3, 6, or 12 h, as indicated. The TSP-1 band is marked with an arrow, and the locations of 28S and 18S ribosomal RNA are shown as reference points. (B) Cells were treated with 10 nM E2 for 6 h in the presence of actinomycin D (Act D; 2 μg/ml) or puromycin (PuRo; 10 μg/ml). (C) Cells were treated for 6 h with 10 nM E2, 10 nM E2 in the presence of 1 μM ICI 182,780 (E2 + ICI), or 1 μM ICI 182,780 alone (ICI). (D) Cells were incubated for 6 h with the indicated dose of E2, and TSP-1 message was visualized as shown in panel B. Autoradiograms were scanned and the densitometric values for the TSP-1 bands were normalized to the densitometric values for 28S in each lane. Values are mean + SEM (n = 3 per dose). * Significantly different from control (p < 0.05; Student t-test). (E) Western blot analysis of serum free media collected after treating T47-D cells with estradiol (E2) in the absence (control) and presence or absence of ICI 182,780 (ICI). Treatment of media prior to western blot analysis is described in Methods.
Figure 1. E2 induces TSP-1 message and protein in breast cancer cells
T47-D and MCF-7 cells were cultured and total RNA prepared and assessed for the expression of TSP-1 mRNA by Northern blot analysis. The blots were then stripped and probed for 28S RNA as a loading control. (A) T47-D cells were treated with 10 nM E2 for 1, 3, 6, or 12 h, as indicated. The TSP-1 band is marked with an arrow, and the locations of 28S and 18S ribosomal RNA are shown as reference points. (B) Cells were treated with 10 nM E2 for 6 h in the presence of actinomycin D (Act D; 2 μg/ml) or puromycin (PuRo; 10 μg/ml). (C) Cells were treated for 6 h with 10 nM E2, 10 nM E2 in the presence of 1 μM ICI 182,780 (E2 + ICI), or 1 μM ICI 182,780 alone (ICI). (D) Cells were incubated for 6 h with the indicated dose of E2, and TSP-1 message was visualized as shown in panel B. Autoradiograms were scanned and the densitometric values for the TSP-1 bands were normalized to the densitometric values for 28S in each lane. Values are mean + SEM (n = 3 per dose). * Significantly different from control (p < 0.05; Student t-test). (E) Western blot analysis of serum free media collected after treating T47-D cells with estradiol (E2) in the absence (control) and presence or absence of ICI 182,780 (ICI). Treatment of media prior to western blot analysis is described in Methods.
Figure 2. The TSP-1 promoter contains an estrogen-responsive region
Reporter gene constructs are shown schematically in the upper part of the figure. The DNA sequence coordinates of the human TSP-1 promoter are indicated; +1 indicates the transcription start site of the TSP-1 gene. Cells were treated with 10 nM E2 ± 1 μM ICI 182,780 (I) for 18 h. Cells were harvested and luciferase activity was quantified. pGL3, empty vector; ERE, estrogen response element from the vitellogenin gene; * significantly different from control; ** Significantly different than E2 treatment in the absence of ICI 182,780 (p < 0.05, Student _t_-test, n = 3 per treatment).
Figure 3. E2 causes release of TSP-1 protein from breast cancer cells
T47-D or MCF-7 cells were incubated overnight in DMEM/F12 + 5% charcoal stripped FBS. Fresh serum-free media was added and cells were incubated for 24 h. The media was then replaced with either serum free DMEM/F12 (T47-D) or DMEM/F12 with 1% charcoal stripped serum (MCF-7) and cells treated with 10 nM E2 (E2-10), an ER-alpha–specific ligand (PPT; 10 nM), or an ER-beta–specific ligand (DPN; 10 nM) ± 1 μM ICI 182,780 (ICI) for 18 h as indicated. The media were collected, and TSP-1 was quantified by ELISA. * significantly different from control; ** significantly different from hormone treatment in the absence of ICI 182,780 (p < 0.05 ANOVA).
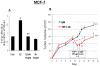
Figure 4. TSP-1-induced proliferation of breast cancer cells
(A) MCF-7 cells were cultured in DME/F12 with 5% DCC serum. The medium was changed to serum-free medium, and the cells were treated for 24 h in the presence of E2 alone, E2 + an antibody against TSP-1 (E2 + Ab), or Ab alone. BrdU was added to the media, and the cells were incubated for an additional 3 h. Cells were then harvested and BrdU was quantified by ELISA. * significantly different from control; ** significantly different from E2 treated group (p < 0.05 ANOVA). (B) MCF-7 xenografts were grown in intact nude mice supplemented with an E2 pellet as described in the Methods. On day 9, the mice were treated with an antibody against TSP-1 or an IgM control. Additional antibody injections were given on days 11, 12, and 13. * Significantly different from IgM-treated control animals (p < 0.05, student t-test).
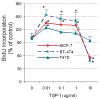
Figure 5. Effect of TSP-1 on proliferation of breast cancer cells
(A) Cells were serum-starved and then incubated in fresh medium without serum with the indicated concentrations of exogenous TSP-1 for 15 h. BrdU was added to the media, and the cells were incubated for an additional 3 h. The cells were harvested, and BrdU was quantified by ELISA. (B) The effect of TSP-1 on breast cancer cell viability. Cells were seeded into 96-well plates overnight as described in Methods. The medium was removed and cells were washed once with DMEM/F12, and treated with identical concentrations shown in (A) in serum free DMEM/F12 medium for 24 hrs. Cell growth and viability were determined by SRB assay as described in Methods. (B) Specificity of TSP-1 effects on breast cancer cells. Cells were cultured as above and treated with either 0.01 or 0.1 μg/ml TSP-1 ± an antibody against TSP-1 (Ab) for 18 h. BrdU was added to the media 3 h prior to termination of the experiment (at 15h). Cells were harvested and BrdU was quantified by ELISA. * Significantly different from control; ** significantly different from hormone treatment in the absence of Ab (p < 0.05, ANOVA).
Figure 5. Effect of TSP-1 on proliferation of breast cancer cells
(A) Cells were serum-starved and then incubated in fresh medium without serum with the indicated concentrations of exogenous TSP-1 for 15 h. BrdU was added to the media, and the cells were incubated for an additional 3 h. The cells were harvested, and BrdU was quantified by ELISA. (B) The effect of TSP-1 on breast cancer cell viability. Cells were seeded into 96-well plates overnight as described in Methods. The medium was removed and cells were washed once with DMEM/F12, and treated with identical concentrations shown in (A) in serum free DMEM/F12 medium for 24 hrs. Cell growth and viability were determined by SRB assay as described in Methods. (B) Specificity of TSP-1 effects on breast cancer cells. Cells were cultured as above and treated with either 0.01 or 0.1 μg/ml TSP-1 ± an antibody against TSP-1 (Ab) for 18 h. BrdU was added to the media 3 h prior to termination of the experiment (at 15h). Cells were harvested and BrdU was quantified by ELISA. * Significantly different from control; ** significantly different from hormone treatment in the absence of Ab (p < 0.05, ANOVA).
Figure 5. Effect of TSP-1 on proliferation of breast cancer cells
(A) Cells were serum-starved and then incubated in fresh medium without serum with the indicated concentrations of exogenous TSP-1 for 15 h. BrdU was added to the media, and the cells were incubated for an additional 3 h. The cells were harvested, and BrdU was quantified by ELISA. (B) The effect of TSP-1 on breast cancer cell viability. Cells were seeded into 96-well plates overnight as described in Methods. The medium was removed and cells were washed once with DMEM/F12, and treated with identical concentrations shown in (A) in serum free DMEM/F12 medium for 24 hrs. Cell growth and viability were determined by SRB assay as described in Methods. (B) Specificity of TSP-1 effects on breast cancer cells. Cells were cultured as above and treated with either 0.01 or 0.1 μg/ml TSP-1 ± an antibody against TSP-1 (Ab) for 18 h. BrdU was added to the media 3 h prior to termination of the experiment (at 15h). Cells were harvested and BrdU was quantified by ELISA. * Significantly different from control; ** significantly different from hormone treatment in the absence of Ab (p < 0.05, ANOVA).
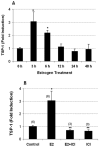
Figure 6. E2-induced TSP-1 message in normal mouse mammary gland
(A) Ovariectomized Balb/c mice (n = 3 per time point) were injected with 40 μg/kg E2 and sacrificed at the times shown. RNA was prepared as described in Methods. TSP-1 message was quantified using real-time PCR with 18S as an internal control. (B) ICI 182,780 (ICI) inhibits estradiol-induced TSP-1 in mouse mammary gland. Ovariectomized mice were injected with ICI (3 mg/kg) 30 min prior to the administration of E2. The mice were sacrificed 3 h later, and RNA was prepared using a Qiagen RNeasy kit. TSP-1 message was quantified using real-time PCR analysis with 18S as an internal control. Numbers in parentheses refer to the number of animals used. * Significantly different from control (p < 0.05; ANOVA).
Similar articles
- WISP-2 gene in human breast cancer: estrogen and progesterone inducible expression and regulation of tumor cell proliferation.
Banerjee S, Saxena N, Sengupta K, Tawfik O, Mayo MS, Banerjee SK. Banerjee S, et al. Neoplasia. 2003 Jan-Feb;5(1):63-73. doi: 10.1016/s1476-5586(03)80018-0. Neoplasia. 2003. PMID: 12659671 Free PMC article. - Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines.
Pink JJ, Jordan VC. Pink JJ, et al. Cancer Res. 1996 May 15;56(10):2321-30. Cancer Res. 1996. PMID: 8625307 - EM-652 (SCH 57068), a third generation SERM acting as pure antiestrogen in the mammary gland and endometrium.
Labrie F, Labrie C, Bélanger A, Simard J, Gauthier S, Luu-The V, Mérand Y, Giguere V, Candas B, Luo S, Martel C, Singh SM, Fournier M, Coquet A, Richard V, Charbonneau R, Charpenet G, Tremblay A, Tremblay G, Cusan L, Veilleux R. Labrie F, et al. J Steroid Biochem Mol Biol. 1999 Apr-Jun;69(1-6):51-84. doi: 10.1016/s0960-0760(99)00065-5. J Steroid Biochem Mol Biol. 1999. PMID: 10418981 Review. - Regulation of mRNA translation by estrogen receptor in breast cancer.
Fard SS, Holz MK. Fard SS, et al. Steroids. 2023 Dec;200:109316. doi: 10.1016/j.steroids.2023.109316. Epub 2023 Oct 6. Steroids. 2023. PMID: 37806603 Free PMC article. Review.
Cited by
- Characterizing Endocrine Status, Tumor Hypoxia and Immunogenicity for Therapy Success in Epithelial Ovarian Cancer.
Pereira M, Matuszewska K, Jamieson C, Petrik J. Pereira M, et al. Front Endocrinol (Lausanne). 2021 Nov 17;12:772349. doi: 10.3389/fendo.2021.772349. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34867818 Free PMC article. Review. - Evaluation of GWAS candidate susceptibility loci for uterine leiomyoma in the multi-ethnic NIEHS uterine fibroid study.
Aissani B, Zhang K, Wiener H. Aissani B, et al. Front Genet. 2015 Jul 14;6:241. doi: 10.3389/fgene.2015.00241. eCollection 2015. Front Genet. 2015. PMID: 26236334 Free PMC article. - Extracellular matrix proteins modulate antimigratory and apoptotic effects of Doxorubicin.
Said G, Guilbert M, Morjani H, Garnotel R, Jeannesson P, El Btaouri H. Said G, et al. Chemother Res Pract. 2012;2012:268681. doi: 10.1155/2012/268681. Epub 2012 Jul 1. Chemother Res Pract. 2012. PMID: 22811904 Free PMC article. - Intervenolin suppresses gastric cancer cell growth through the induction of TSP-1 secretion from fibroblast-like stromal cells.
Yoshida J, Abe H, Watanabe T, Kawada M. Yoshida J, et al. Oncol Lett. 2018 Nov;16(5):6777-6785. doi: 10.3892/ol.2018.9485. Epub 2018 Sep 21. Oncol Lett. 2018. PMID: 30405822 Free PMC article. - Advancement of mass spectrometry-based proteomics technologies to explore triple negative breast cancer.
Miah S, Banks CA, Adams MK, Florens L, Lukong KE, Washburn MP. Miah S, et al. Mol Biosyst. 2016 Dec 20;13(1):42-55. doi: 10.1039/c6mb00639f. Mol Biosyst. 2016. PMID: 27891540 Free PMC article. Review.
References
- Sid B, Sartelet H, Bellon G, El Btaouri H, Rath G, Delorme N, Haye B, Martiny L. Thrombospondin 1: a multifunctional protein implicated in the regulation of tumor growth. Crit Rev Oncol Hematol. 2004;49:245–8. - PubMed
- Wang TN, Qian XH, Granick MS, Solomon MP, Rothman VL, Berger DH, Tuszynski GP. Inhibition of breast cancer progression by an antibody to a thrombospondin-1 receptor. Surgery. 1996;120:449–54. - PubMed
- Wang TN, Qian X, Granick MS, Solomon MP, Rothman VL, Berger DH, Tuszynski GP. Thrombospondin-1 (TSP-1) promotes the invasive properties of human breast cancer. J Surg Res. 1996;63:39–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous