Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival - PubMed (original) (raw)
Review
Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival
Kuniyasu Niizuma et al. J Neurochem. 2009 May.
Abstract
Mitochondria are the powerhouse of the cell. Their primary physiological function is to generate adenosine triphosphate through oxidative phosphorylation via the electron transport chain. Reactive oxygen species generated from mitochondria have been implicated in acute brain injuries such as stroke and neurodegeneration. Recent studies have shown that mitochondrially-formed oxidants are mediators of molecular signaling, which is implicated in the mitochondria-dependent apoptotic pathway that involves pro- and antiapoptotic protein binding, the release of cytochrome c, and transcription-independent p53 signaling, leading to neuronal death. Oxidative stress and the redox state of ischemic neurons are also implicated in the signaling pathway that involves phosphatidylinositol 3-kinase/Akt and downstream signaling, which lead to neuronal survival. Genetically modified mice or rats that over-express or are deficient in superoxide dismutase have provided strong evidence in support of the role of mitochondrial dysfunction and oxidative stress as determinants of neuronal death/survival after stroke and neurodegeneration.
Figures
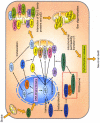
Fig. 1
Involvement of p53 signaling after ROS generation. After ROS generation from mitochondria, p53 transcriptionally generates pro-apoptotic proteins such as Bax, Noxa, p53AIP1, PUMA, and Bid. These products act directly on mitochondria. Mitochondrial translocation of Bax is promoted by JNK through transcriptional activation of Bim. Full-length PIDD (PIDD-FL) is also transcriptionally upregulated by p53. PIDD-CC, a fragment of PIDD-FL cleaved by autoproteolysis, activates caspase-2 through the formation of the PIDDosome, which precedes Bid truncation and translocation to mitochondria. Moreover, p53 translocates to the mitochondrial membrane and activates the mitochondria-dependent apoptotic pathway in a transcription-independent manner. BH3-only proteins and p53 interact with both pro-apoptotic Bax and anti-apoptotic Bcl-XL on the mitochondrial membrane. This interaction causes Bax oligomerization and activation, which triggers cytochrome c release, leading to neuronal death. tBid, truncated Bid.
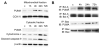
Fig. 2
Mitochondrial PUMA upregulation after tGCI. (A) Western blot analysis shows that mitochondrial PUMA increased 4 and 24 h after tGCI, followed by cytosolic upregulation of cleaved caspase-9 and cytochrome c release. β-actin and cytochrome oxidase subunit IV (COX IV) analyses are shown as internal controls. c, control. (B) Coimmunoprecipitation analyses show that PUMA immunoreactivity precipitated by Bcl-XL or Bax increased after tGCI. Bcl-XL precipitated by Bcl-XL, and Bax precipitated by Bax were used to show equal precipitation. IP, immunoprecipitation; IB, immunoblotting. (Data modified from Niizuma et al. 2009.)
Similar articles
- Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke.
Chan PH. Chan PH. Ann N Y Acad Sci. 2005 May;1042:203-9. doi: 10.1196/annals.1338.022. Ann N Y Acad Sci. 2005. PMID: 15965064 - Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia.
Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Chen SD, et al. Int J Mol Sci. 2011;12(10):7199-215. doi: 10.3390/ijms12107199. Epub 2011 Oct 21. Int J Mol Sci. 2011. PMID: 22072942 Free PMC article. Review. - Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia.
Chan PH. Chan PH. Neurochem Res. 2004 Nov;29(11):1943-9. doi: 10.1007/s11064-004-6869-x. Neurochem Res. 2004. PMID: 15662830 Review. - Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.
Ferrer I, Planas AM. Ferrer I, et al. J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review. - Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation.
Sugawara T, Noshita N, Lewén A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Sugawara T, et al. J Neurosci. 2002 Jan 1;22(1):209-17. doi: 10.1523/JNEUROSCI.22-01-00209.2002. J Neurosci. 2002. PMID: 11756504 Free PMC article.
Cited by
- Peroxisome proliferator-activated receptor-gamma dependent pathway reduces the phosphorylation of dynamin-related protein 1 and ameliorates hippocampal injury induced by global ischemia in rats.
Chuang YC, Lin TK, Yang DI, Yang JL, Liou CW, Chen SD. Chuang YC, et al. J Biomed Sci. 2016 May 12;23(1):44. doi: 10.1186/s12929-016-0262-3. J Biomed Sci. 2016. PMID: 27175924 Free PMC article. - Influence of physical exercise on traumatic brain injury deficits: scaffolding effect.
Archer T. Archer T. Neurotox Res. 2012 May;21(4):418-34. doi: 10.1007/s12640-011-9297-0. Epub 2011 Dec 20. Neurotox Res. 2012. PMID: 22183422 Review. - Association between bilirubin levels with incidence and prognosis of stroke: A meta-analysis.
Zhao K, Wang R, Chen R, Liu J, Ye Q, Wang K, Li J. Zhao K, et al. Front Neurosci. 2023 Feb 14;17:1122235. doi: 10.3389/fnins.2023.1122235. eCollection 2023. Front Neurosci. 2023. PMID: 36866331 Free PMC article. - Leonurine promotes neurite outgrowth and neurotrophic activity by modulating the GR/SGK1 signaling pathway in cultured PC12 cells.
Meng P, Zhu Q, Yang H, Liu D, Lin X, Liu J, Fan J, Liu X, Su W, Liu L, Wang Y, Cai X. Meng P, et al. Neuroreport. 2019 Mar 6;30(4):247-254. doi: 10.1097/WNR.0000000000001180. Neuroreport. 2019. PMID: 30694908 Free PMC article.
References
- Bennett M, Macdonald K, Chan S-W, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290–293. - PubMed
- Caelles C, Helmberg A, Karin M. p53-Dependent apoptosis in the absence of transcriptional activation of p53-target genes [Letter] Nature. 1994;370:220–223. - PubMed
- Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS038653/NS/NINDS NIH HHS/United States
- R01 NS025372-21/NS/NINDS NIH HHS/United States
- R01 NS038653-11A1/NS/NINDS NIH HHS/United States
- R01 NS036147-12/NS/NINDS NIH HHS/United States
- P50 NS014543/NS/NINDS NIH HHS/United States
- P50 NS014543-29/NS/NINDS NIH HHS/United States
- R01 NS025372/NS/NINDS NIH HHS/United States
- R01 NS036147/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous