Endobrevin/VAMP-8-dependent dense granule release mediates thrombus formation in vivo - PubMed (original) (raw)
Endobrevin/VAMP-8-dependent dense granule release mediates thrombus formation in vivo
Gwenda J Graham et al. Blood. 2009.
Abstract
Individuals whose platelets lack dense or alpha-granules suffer various degrees of abnormal bleeding, implying that granule cargo contributes to hemostasis. Despite these clinical observations, little is known regarding the effects of impaired platelet granule secretion on thrombus formation in vivo. In platelets, SNARE proteins mediate the membrane fusion events required for granule cargo release. Endobrevin/VAMP-8 is the primary vesicle-SNARE (v-SNARE) responsible for efficient release of dense and alpha-granule contents; thus, VAMP-8(-/-) mice are a useful model to evaluate the importance of platelet granule secretion in thrombus formation. Thrombus formation, after laser-induced vascular injury, in these mice is delayed and decreased, but not absent. In contrast, thrombus formation is almost completely abolished in the mouse model of Hermansky-Pudlak syndrome, ruby-eye, which lacks dense granules. Evaluation of aggregation of VAMP-8(-/-) and ruby-eye platelets indicates that defective ADP release is the primary abnormality leading to impaired aggregation. These results demonstrate the importance of dense granule release even in the earliest phases of thrombus formation and validate the distal platelet secretory machinery as a potential target for antiplatelet therapies.
Figures
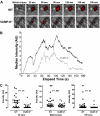
Figure 1
Platelet accumulation in VAMP-8−/− mice after laser-induced injury of cremaster arterioles. (A) Platelet accumulation was imaged at the indicated time intervals after injury in both wild-type (WT) and VAMP-8–deficient (VAMP-8_−/−)_ mice. (B) Median integrated platelet fluorescence intensity was plotted at each second for 180 seconds after laser injury in VAMP-8−/− mice. (C) At 30 seconds, median platelet accumulation in VAMP-8−/− mice was 4% of WT (P = .01). There was a trend toward a decrease in maximal thrombus size in VAMP-8−/− mice compared with WT mice, but the difference was not statistically significant (74% of WT; P = .061). By 180 seconds, median platelet accumulation in VAMP-8−/− mice was 28% of WT mice (P = .01).
Figure 2
Role of ADP in aggregation of VAMP-8−/− platelets. Wild-type (A) and VAMP-8−/− (B) platelets were incubated with the indicated concentrations of thrombin and percentage of light transmission was recorded. Panel C represents the mean percentage of aggregation versus thrombin concentration with SD indicated (3 aggregations/condition, n = 8 mice). Thrombin (D: 15 mU/mL; E: 100 mU/mL) was used to stimulate WT (black line) or VAMP-8−/− platelets (gray line). Aggregation (upper trace) and release of ATP (lower trace) were monitored. (F) Aggregations were measured for VAMP-8−/− platelets stimulated with 15 mU/mL thrombin and wild-type platelets stimulated with 15 mU/mL thrombin plus 1 U/mL apyrase. (G) Aggregations were measured for VAMP-8−/− platelets stimulated with 15 mU/mL thrombin alone, 15 mU/mL thrombin plus 2 μM ADP, or 2 μM ADP alone. All aggregation measurements were performed with constant stirring.
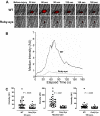
Figure 3
Platelet accumulation in ruby-eye mice after laser-induced injury of cremaster arterioles. (A) Platelet accumulation was imaged at the indicated time intervals, after injury, in both wild-type (WT) and ruby-eye mice. (B) Median integrated platelet fluorescence intensity was recorded at each second for 180 seconds after laser injury in ruby-eye mice. (C) At 30 seconds, median platelet accumulation in ruby-eye mice was 22% of WT (P = .001). Maximal thrombus size in ruby-eye mice was decreased compared with WT mice (12% of WT; P = .001). By 180 seconds, median platelet accumulation in ruby-eye mice was 31% of WT mice (P = .007).
Figure 4
Thrombin-mediated aggregation of ruby-eye platelets. Wild-type (A) and ruby-eye (B) platelets were incubated with the indicated concentrations of thrombin and percentage of light transmission was recorded. (C) Mean percentage aggregation in wild-type and ruby-eye platelets was plotted as a function of thrombin concentration with standard deviation indicated (n = 3-8 aggregations/condition, n = 5 mice). (D) Ruby-eye platelets were incubated with 2 μM ADP alone, 25 mU/mL thrombin alone, or 25 mU/mL thrombin plus 2 μM ADP as indicated. Platelet aggregation was measured with constant stirring.
Comment in
- Granules and thrombus formation.
Kahr WH. Kahr WH. Blood. 2009 Jul 30;114(5):932-3. doi: 10.1182/blood-2009-05-220665. Blood. 2009. PMID: 19643993 No abstract available.
Similar articles
- Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome.
Sharda A, Kim SH, Jasuja R, Gopal S, Flaumenhaft R, Furie BC, Furie B. Sharda A, et al. Blood. 2015 Mar 5;125(10):1633-42. doi: 10.1182/blood-2014-08-597419. Epub 2015 Jan 15. Blood. 2015. PMID: 25593336 Free PMC article. - Defective release of α granule and lysosome contents from platelets in mouse Hermansky-Pudlak syndrome models.
Meng R, Wu J, Harper DC, Wang Y, Kowalska MA, Abrams CS, Brass LF, Poncz M, Stalker TJ, Marks MS. Meng R, et al. Blood. 2015 Mar 5;125(10):1623-32. doi: 10.1182/blood-2014-07-586727. Epub 2014 Dec 4. Blood. 2015. PMID: 25477496 Free PMC article. - VAMP-7 links granule exocytosis to actin reorganization during platelet activation.
Koseoglu S, Peters CG, Fitch-Tewfik JL, Aisiku O, Danglot L, Galli T, Flaumenhaft R. Koseoglu S, et al. Blood. 2015 Jul 30;126(5):651-60. doi: 10.1182/blood-2014-12-618744. Epub 2015 May 21. Blood. 2015. PMID: 25999457 Free PMC article. - Sorting machineries: how platelet-dense granules differ from α-granules.
Chen Y, Yuan Y, Li W. Chen Y, et al. Biosci Rep. 2018 Sep 7;38(5):BSR20180458. doi: 10.1042/BSR20180458. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 30104399 Free PMC article. Review. - Molecular basis of platelet granule defects.
Yao HHY, Kahr WHA. Yao HHY, et al. J Thromb Haemost. 2025 Feb;23(2):381-393. doi: 10.1016/j.jtha.2024.11.016. Epub 2024 Nov 29. J Thromb Haemost. 2025. PMID: 39617187 Review.
Cited by
- Mechanism of platelet α-granule biogenesis: study of cargo transport and the VPS33B-VPS16B complex in a model system.
Ambrosio AL, Di Pietro SM. Ambrosio AL, et al. Blood Adv. 2019 Sep 10;3(17):2617-2626. doi: 10.1182/bloodadvances.2018028969. Blood Adv. 2019. PMID: 31501156 Free PMC article. - Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome.
Sharda A, Kim SH, Jasuja R, Gopal S, Flaumenhaft R, Furie BC, Furie B. Sharda A, et al. Blood. 2015 Mar 5;125(10):1633-42. doi: 10.1182/blood-2014-08-597419. Epub 2015 Jan 15. Blood. 2015. PMID: 25593336 Free PMC article. - Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis.
Ren Q, Wimmer C, Chicka MC, Ye S, Ren Y, Hughson FM, Whiteheart SW. Ren Q, et al. Blood. 2010 Aug 12;116(6):869-77. doi: 10.1182/blood-2010-02-270934. Epub 2010 Apr 30. Blood. 2010. PMID: 20435885 Free PMC article. - Platelet secretion and hemostasis require syntaxin-binding protein STXBP5.
Ye S, Huang Y, Joshi S, Zhang J, Yang F, Zhang G, Smyth SS, Li Z, Takai Y, Whiteheart SW. Ye S, et al. J Clin Invest. 2014 Oct;124(10):4517-28. doi: 10.1172/JCI75572. Epub 2014 Sep 17. J Clin Invest. 2014. PMID: 25244094 Free PMC article. - The secreted tyrosine kinase VLK is essential for normal platelet activation and thrombus formation.
Revollo L, Merrill-Skoloff G, De Ceunynck K, Dilks JR, Guo S, Bordoli MR, Peters CG, Noetzli L, Ionescu A, Rosen V, Italiano JE, Whitman M, Flaumenhaft R. Revollo L, et al. Blood. 2022 Jan 6;139(1):104-117. doi: 10.1182/blood.2020010342. Blood. 2022. PMID: 34329392 Free PMC article.
References
- Hermansky F, Pudlak P, Mlejnkova M, Spankova H. [Unusual cases of hemorrhagic states from the group of so-called hypothromboplastinamias]. Cas Lek Cesk. 1956;95:182–187. - PubMed
- Hermansky F, Pudlak P. Albinism associated with hemorrhagic diathesis and unusual pigmented reticular cells in the bone marrow: report of two cases with histochemical studies. Blood. 1959;14:162–169. - PubMed
- Logan LJ, Rapaport SI, Maher I. Albinism and abnormal platelet function. N Engl J Med. 1971;284:1340–1345. - PubMed
- Swank RT, Novak EK, McGarry MP, Rusiniak ME, Feng L. Mouse models of Hermansky Pudlak syndrome: a review. Pigment Cell Res. 1998;11:60–80. - PubMed
- Raccuglia G. Gray platelet syndrome: a variety of qualitative platelet disorder. Am J Med. 1971;51:818–828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL56652/HL/NHLBI NIH HHS/United States
- HL63250/HL/NHLBI NIH HHS/United States
- R01 HL063250/HL/NHLBI NIH HHS/United States
- HL091893/HL/NHLBI NIH HHS/United States
- T32 HL07917/HL/NHLBI NIH HHS/United States
- T32 HL007917/HL/NHLBI NIH HHS/United States
- R01 HL056652/HL/NHLBI NIH HHS/United States
- R01 HL091893/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases