Parvalbumin neurons and gamma rhythms enhance cortical circuit performance - PubMed (original) (raw)
. 2009 Jun 4;459(7247):698-702.
doi: 10.1038/nature07991. Epub 2009 Apr 26.
Affiliations
- PMID: 19396159
- PMCID: PMC3969859
- DOI: 10.1038/nature07991
Parvalbumin neurons and gamma rhythms enhance cortical circuit performance
Vikaas S Sohal et al. Nature. 2009.
Abstract
Synchronized oscillations and inhibitory interneurons have important and interconnected roles within cortical microcircuits. In particular, interneurons defined by the fast-spiking phenotype and expression of the calcium-binding protein parvalbumin have been suggested to be involved in gamma (30-80 Hz) oscillations, which are hypothesized to enhance information processing. However, because parvalbumin interneurons cannot be selectively controlled, definitive tests of their functional significance in gamma oscillations, and quantitative assessment of the impact of parvalbumin interneurons and gamma oscillations on cortical circuits, have been lacking despite potentially enormous significance (for example, abnormalities in parvalbumin interneurons may underlie altered gamma-frequency synchronization and cognition in schizophrenia and autism). Here we use a panel of optogenetic technologies in mice to selectively modulate multiple distinct circuit elements in neocortex, alone or in combination. We find that inhibiting parvalbumin interneurons suppresses gamma oscillations in vivo, whereas driving these interneurons (even by means of non-rhythmic principal cell activity) is sufficient to generate emergent gamma-frequency rhythmicity. Moreover, gamma-frequency modulation of excitatory input in turn was found to enhance signal transmission in neocortex by reducing circuit noise and amplifying circuit signals, including inputs to parvalbumin interneurons. As demonstrated here, optogenetics opens the door to a new kind of informational analysis of brain function, permitting quantitative delineation of the functional significance of individual elements in the emergent operation and function of intact neural circuitry.
Figures
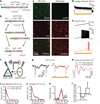
Figure 1. Inhibiting PV cells suppresses gamma oscillations in vivo
a, Double-floxed Cre-dependent AAV vector design. hGH, human growth hormone polyadenylation signal; ITR, inverted terminal repeat; WPRE, woodchuck heptatitis virus post-transcriptional regulatory element. b, Recombination pathways to low-leak Cre-dependent expression. c, Left: antibody-stained PV cells (red) expressing ChR2–eYFP (green) in prefrontal cortex (PFC) of PV::Cre mice after injection of Cre-dependent AAV. Right: absent ChR2–eYFP expression in similarly treated wild-type mice. d, Yellow-light-evoked outward current in eNpHR-expressing PV cells in acute slice. e, Effect of yellow light on current-ramp-evoked spiking in the same cell. f, Experimental design: in vivo local field potentials (LFPs) recorded in mouse PFC; blue and yellow light modulate ChR2(+) PY and eNpHR(+) fast-spiking (FS)/PV cells, respectively. g, Sample blue-light-evoked LFPs; g–k show data from a single location in vivo; red traces denote recordings in yellow light. h, Filtered (35–45 Hz)-light-evoked LFPs. i, Spontaneous LFP power spectra. j, Blue-light-evoked LFP power spectra. k, Yellow light modulation of phase-locking (Supplementary Information). Dotted line denotes statistical significance. l, Effect of fast-spiking cell inhibition on peak power at gamma and lower frequencies (n = 4 recording locations; ***P < 0.001). Error bars, mean and s.e.m.
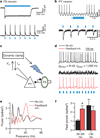
Figure 2. Feedback inhibition from PV cells generates emergent gamma frequency synchrony
a, Light-evoked responses in a fast-spiking PV interneuron (FS). b, Responses of a ChR2(–) PY cell during photoactivation of ChR2(+) PV interneurons. c, Experimental design: sEPSCs drive PY cells. Light flashes triggered by PY cell spikes activate FS/PV interneurons (optical feedback inhibition). _V_m, measured membrane potential; _I_m, injected current. d, PY cell responses to non-rhythmic sEPSCs with and without this optical feedback inhibition (inh.). _g_EPSC, unitary sEPSC conductance; _f_EPSC, sEPSC frequency. e, Power spectra obtained by convolving the spike trains of a PY cell with wavelets of varying frequencies; red trace represents optical feedback inhibition via PV interneurons. f, Summary of spectral data at gamma (30–80 Hz) and lower frequencies (n = 4 cells; *P < 0.05). Error bars, mean and s.e.m.
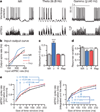
Figure 3. Gamma oscillations amplify signals and reduce noise in PY cells
a, PY cell responses (top traces) to non-rhythmic (NR), repeated non-rhythmic (Rep), or rhythmic defined sEPSC trains (lower traces; Supplementary Information; depicted rates = 40–372 Hz). b, Spike rates of representative PY cell under each rhythmicity condition. c, Maximum input–output (I–O) gain. d, Response variability for each condition. e, Left: mutual information between output spike number and input sEPSCs; gamma oscillations consistently enhanced mutual information. Right: contrasting effect on sEPSC spike rate information in simulated integrate and-fire cells using the same sEPSC trains (n = 14 cells in c–e; ***P < 0.001). Key in e is the same as for b. Error bars, mean and s.e.m.
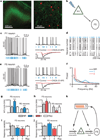
Figure 4. Gamma oscillations enhance information flow from PY to PV cells
a, eYFP (green) and PV (red) cells in layer V PFC of _Thy1_–ChR2–eYFP transgenic mice. b, Experimental design: light flashes excite PY neurons, which synaptically excite PV cells. c, Light directly excites PY cells. Current evoked by 1-ms flashes with/without synaptic blockers (CNQX and D-AP5). d, Responses of PY neurons from different slices to the same light train (blue). e, Light indirectly excites fast-spiking (FS) interneurons. Current evoked by 1-ms flashes with/without synaptic blockers. f, Power spectra of PY neuron spiking elicited by distinct-rhythmicity light trains. g, h, Mutual information (Inf) between responses of fast-spiking or regular-spiking (RS) neurons and PY neurons to the same rhythmic or non-rhythmic light trains (Supplementary Information). i, j, Response variability of fast-spiking and regular-spiking cells for rhythmic and non-rhythmic stimuli (n = 12 PY, 7 fast-spiking, and 9 regular-spiking cells). k, Interplay of gamma (γ) oscillations and fast-spiking neurons. Error bars, mean and s.e.m.
Similar articles
- Myelination synchronizes cortical oscillations by consolidating parvalbumin-mediated phasic inhibition.
Dubey M, Pascual-Garcia M, Helmes K, Wever DD, Hamada MS, Kushner SA, Kole MHP. Dubey M, et al. Elife. 2022 Jan 10;11:e73827. doi: 10.7554/eLife.73827. Elife. 2022. PMID: 35001871 Free PMC article. - Parvalbumin and Somatostatin Interneurons Contribute to the Generation of Hippocampal Gamma Oscillations.
Antonoudiou P, Tan YL, Kontou G, Upton AL, Mann EO. Antonoudiou P, et al. J Neurosci. 2020 Sep 30;40(40):7668-7687. doi: 10.1523/JNEUROSCI.0261-20.2020. Epub 2020 Aug 28. J Neurosci. 2020. PMID: 32859716 Free PMC article. - Insights into cortical oscillations arising from optogenetic studies.
Sohal VS. Sohal VS. Biol Psychiatry. 2012 Jun 15;71(12):1039-45. doi: 10.1016/j.biopsych.2012.01.024. Epub 2012 Mar 3. Biol Psychiatry. 2012. PMID: 22381731 Free PMC article. Review. - Contribution of parvalbumin and somatostatin-expressing GABAergic neurons to slow oscillations and the balance in beta-gamma oscillations across cortical layers.
Kuki T, Fujihara K, Miwa H, Tamamaki N, Yanagawa Y, Mushiake H. Kuki T, et al. Front Neural Circuits. 2015 Feb 3;9:6. doi: 10.3389/fncir.2015.00006. eCollection 2015. Front Neural Circuits. 2015. PMID: 25691859 Free PMC article. - Transforming Discoveries About Cortical Microcircuits and Gamma Oscillations Into New Treatments for Cognitive Deficits in Schizophrenia.
Sohal VS. Sohal VS. Am J Psychiatry. 2022 Apr;179(4):267-276. doi: 10.1176/appi.ajp.20220147. Am J Psychiatry. 2022. PMID: 35360913 Review.
Cited by
- Atypical laterality of resting gamma oscillations in autism spectrum disorders.
Maxwell CR, Villalobos ME, Schultz RT, Herpertz-Dahlmann B, Konrad K, Kohls G. Maxwell CR, et al. J Autism Dev Disord. 2015 Feb;45(2):292-7. doi: 10.1007/s10803-013-1842-7. J Autism Dev Disord. 2015. PMID: 23624928 Free PMC article. - Enhancement of gamma activity after selective activation of dopamine D4 receptors in freely moving rats and in a neurodevelopmental model of schizophrenia.
Kocsis B, Lee P, Deth R. Kocsis B, et al. Brain Struct Funct. 2014 Nov;219(6):2173-80. doi: 10.1007/s00429-013-0607-6. Epub 2013 Jul 10. Brain Struct Funct. 2014. PMID: 23839116 Free PMC article. - Cell-Specific Targeting of Genetically Encoded Tools for Neuroscience.
Sjulson L, Cassataro D, DasGupta S, Miesenböck G. Sjulson L, et al. Annu Rev Genet. 2016 Nov 23;50:571-594. doi: 10.1146/annurev-genet-120215-035011. Epub 2016 Oct 6. Annu Rev Genet. 2016. PMID: 27732792 Free PMC article. Review. - Role of neuropsin in parvalbumin immunoreactivity changes in hippocampal basket terminals of mice reared in various environments.
Suzuki H, Kanagawa D, Nakazawa H, Tawara-Hirata Y, Kogure Y, Shimizu-Okabe C, Takayama C, Ishikawa Y, Shiosaka S. Suzuki H, et al. Front Cell Neurosci. 2014 Dec 10;8:420. doi: 10.3389/fncel.2014.00420. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25540610 Free PMC article. - Contribution of synchronized GABAergic neurons to dopaminergic neuron firing and bursting.
Morozova EO, Myroshnychenko M, Zakharov D, di Volo M, Gutkin B, Lapish CC, Kuznetsov A. Morozova EO, et al. J Neurophysiol. 2016 Oct 1;116(4):1900-1923. doi: 10.1152/jn.00232.2016. Epub 2016 Jul 20. J Neurophysiol. 2016. PMID: 27440240 Free PMC article.
References
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. - PubMed
- Toledo-Rodriguez M, et al. Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb. Cortex. 2004;14:1310–1327. - PubMed
- Freund TF. Interneuron diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. - PubMed
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. - PubMed
- Ylinen A, et al. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus. 1995;5:78–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials