RAD18 transmits DNA damage signalling to elicit homologous recombination repair - PubMed (original) (raw)
. 2009 May;11(5):592-603.
doi: 10.1038/ncb1865. Epub 2009 Apr 26.
Affiliations
- PMID: 19396164
- PMCID: PMC2743127
- DOI: 10.1038/ncb1865
RAD18 transmits DNA damage signalling to elicit homologous recombination repair
Jun Huang et al. Nat Cell Biol. 2009 May.
Abstract
To maintain genome stability, cells respond to DNA damage by activating signalling pathways that govern cell-cycle checkpoints and initiate DNA repair. Cell-cycle checkpoint controls should connect with DNA repair processes, however, exactly how such coordination occurs in vivo is largely unknown. Here we describe a new role for the E3 ligase RAD18 as the integral component in translating the damage response signal to orchestrate homologous recombination repair (HRR). We show that RAD18 promotes homologous recombination in a manner strictly dependent on its ability to be recruited to sites of DNA breaks and that this recruitment relies on a well-defined DNA damage signalling pathway mediated by another E3 ligase, RNF8. We further demonstrate that RAD18 functions as an adaptor to facilitate homologous recombination through direct interaction with the recombinase RAD51C. Together, our data uncovers RAD18 as a key factor that orchestrates HRR through surveillance of the DNA damage signal.
Figures
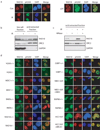
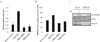
Figure 1. RAD18 forms DNA double-strand break-induced foci
(a) Localization of RAD18 in response to IR or CPT. HeLa cells were treated with 10 Gy of ionizing radiation or 1 µM CPT, fixed and immunostained with anti-RAD18 and pH2AX antibodies. (b, c) RAD18 relocalizes to chromatin fraction after IR treatment (b), which is reversible following micrococcal nuclease treatment (c). Experiments were carried out as described in the Experimental Procedures and immunoblotting experiments were performed using indicated antibodies. (d) Genetic dependence of RAD18 relocalization following IR treatment. Indicated deficient cells and their respective wild-type counterparts were irradiated, fixed and immunostained with anti-RAD18 and pH2AX antibodies.
Figure 2. RNF8/UBC13 is required for DSB-induced RAD18 recruitment
(a) Both the FHA and RING domain of RNF8 are required for RAD18 relocalization. RNF8 deficient cells were reconstituted with wild-type or different internal deletion mutants of RNF8. The expression of wild-type and mutant RNF8 was confirmed by Western blotting (lower panel). Following irradiation, cells were fixed and immunostained using anti-RAD18 and anti-Flag antibodies. Representative RAD18 foci were shown in the upper panel. Results were the average of three independent experiments in which more than one hundred cells were counted per experiment and were presented as mean±SEM (lower panel). (b) UBC13 is required for RAD18 relocalization. Wild-type or UBC13 deficient cells were irradiated, fixed and immunostained with anti-RAD18 and anti-pH2AX antibodies. (c) RNF8 is required for IR-induced RAD18 chromatin localization. HeLa cells depleted of endogenous RAD18 or RNF8 were treated with 10 Gy IR or left untreated. Six hours later, cells were collected and chromatin fractions were isolated according to Experimental Procedures. Immunoblotting experiments were performed using indicated antibodies. (d) RAD18 is not required for IR-induced ubiquitin conjugate formation. Wild-type, RAD18 or RNF8 deficient cells were irradiated, fixed and immunostained with anti-FK2 and pH2AX antibodies. (e) RAD18 is not required for H2AX ubiquitination following DNA damage. HeLa cells transfected with control siRNA, RAD18 siRNA or RNF8 siRNA were treated with 10 Gy IR or left untreated. Cells were harvested one hour post-irradiation and cell lysates were immunoblotted with indicated antibodies. (f) RAD18 is not required for Rap80 and BRCA1 foci formation following IR. Wild-type or RAD18 deficient cells were irradiated, fixed and immunostained with anti-Rap80 or BRCA1 antibodies.
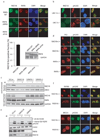
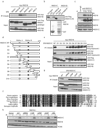
Figure 3. The ZNF domain of RAD18 is required for RAD18 localization to the sites of DNA damage
(a) Schematic representation of human RAD18 and its deletion/point mutants used in this study. (b) The ZNF domain of RAD18 targets it to IR-induced foci. 293T cells expressing Flag-tagged wild-type or mutants of RAD18 were irradiated, fixed and immunostained with anti-Flag and pH2AX antibodies. (c) The ZNF domain alone is sufficient for RAD18 IRIF. 293T cells expressing indicated Flag-tagged proteins were irradiated and immunostained as described in (b). (d) Genetic dependence of the ZNF domain relocalization following ionizing radiation. Immunostaining experiments were performed as described in Figure 1d. (e, f) The ZNF domain of RAD18 is essential and sufficient for binding to ubiquitin in vitro. GST or Ubi-GST fusion proteins were incubated with cell lysates containing exogenously expressed Flag-tagged wild-type or various internal deletion mutants of RAD18 (e) or NLS-ZNF or NLS-ZNF-C207F (f). After extensive washing, bound RAD18 fragments or fusion proteins were analyzed by immunoblotting with anti-Flag antibody.
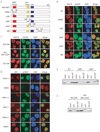
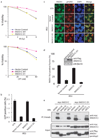
Figure 4. RAD18 promotes homologous recombination
(a) The RAD18 ZNF and RING domains, but not its E3 ligase activity, are required for restoring cellular resistance to DSBs. Colony formation assays were performed according to the Experimental Procedures. Results were averages of three independent experiments and were presented as mean±SEM. (b) RAD18 promotes homologous recombination. RAD18-deficient cells were reconstituted with wild-type or different RAD18 mutants as indicated. Gene conversion assays were performed as described in the Experimental Procedures. Results (mean±SEM) were the average of three independent experiments. (c, d) RAD18 is required for efficient RAD51 foci formation. RAD18-deficient or reconstituted cells were irradiated, fixed and immunostained with anti-RAD51 and pH2AX antibodies. Representative RAD51 foci were shown (c). Data shown in (d) are from three independent experiments in which more than one hundred cells were counted in each experiment. Results were the average of three independent experiments and were presented as mean±SEM.
Figure 5. RNF8 participates in homologous recombination
(a) RNF8 promotes homologous recombination. RNF8-deficient cells were reconstituted with wild-type or different mutants of RNF8 as indicated in Figure 2a. Gene Conversion assay were performed similar to those described in Figure 4b. (b) RNF8 is required for efficient RAD51 foci formation. (c) RAD18 is not required for CHK1 activation following DNA damage. Control or RAD18 siRNA-transfected HeLa cells were mock treated or exposed to 10 Gy of ionizing radiation. Cells were harvested 2 hours later and lystes were immunoblotted with indicated antibodies.
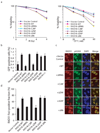
Figure 6. RAD18 interacts with RAD51C
(a) Ectopically expressed RAD18 interacts with RAD51C. 293T cells were transfected with plasmids encoding Myc-tagged RAD18 together with plasmids encoding SFB-tagged RAD51 or RAD51 paralogs. Cells were collected 24 hour after transfection. Immunoprecipitation (IP) reactions were performed using S beads and then subjected to immunoblotting using antibodies as indicated. (b) RAD18 directly interacts with RAD51C. GST or GST-RAD18 immobilized on sepharose beads were incubated with cell lysates containing exogenously expressed Flag-tagged RAD51C or RAD51D. Bound RAD51C was analyzed by anti-Flag immunoblotting. (c) Endogenous RAD18 and RAD51C form a complex in vivo. 293T cells were mock treated or treated with IR (10 Gy). Control or anti-RAD51C immunoprecipitates were immunoblotted with RAD18 and RAD51C antibodies (upper panels). The expression levels of the endogenous proteins were detected by immunoblotting using RAD18 and RAD51C antibodies (lower panels). (d) Schematic representation of human RAD51C and its deletion mutants used in this study. (e, g) Mapping of the corresponding regions required for RAD18/RAD51C interaction. Immunoprecipitation (IP) reactions were performed using S beads and then subjected to immunoblotting using antibodies as indicated. (f) Alignment of RAD51C N-terminal sequences from different species. (h) RAD18 is required for IR-induced RAD51C chromatin localization. RAD18-deficient cells were reconstituted with wild-type or different RAD18 mutants as indicated. Cells were irradiated and chromatin fractions were isolated according to Experimental Procedures. Immunoblotting experiments were performed using indicated antibodies.
Figure 7. RAD18-binding is critical for RAD51C function in homologous recombination
(a) D1 mutant of RAD51C is defective in restoring cell survival following IR or CPT treatment. RAD51C-deficient IRS3 cells were transduced with control virus or viruses expressing HA-Flag-tagged wild-type RAD51C (WT) or the very N-terminal deletion mutant (D1), which does not bind to RAD18. Wild-type or RAD51C-D1 expression was confirmed by immunoblotting as indicated in (d). Clonogenic assays were performed according to the Experimental Procedures and results were averages of three independent experiments and presented as mean±SEM. (b) RAD18-binding is required for RAD51C function in homologous recombination. RAD51C-deficient IRS3 cells were reconstituted with wild-type or D1 mutant of RAD51C. Gene conversion assays were performed as described in Experimental Procedures. Results were the average of three independent experiments and were presented as mean±SEM. (c, d) Interaction with RAD18 is important for RAD51C function in promoting RAD51 foci formation. Cells as indicated were irradiated, fixed and immunostained with anti-RAD51 and pH2AX antibodies. Representative RAD51 foci were shown in (c). Results were the averages of three independent experiments in which more than one hundred cells were counted in every experiment and were presented as mean±SEM (d). (e) The N terminal region of RAD51C is not required for RAD51 paralogs-complexes formation. 293T cells were transfected with plasmids encoding Myc-tagged RAD51C or RAD51C-D1 together with plasmids encoding SFB-tagged RAD51 or RAD51 paralogs. Immunoprecipitation (IP) reactions were performed using S beads and then subjected to immunoblotting using indicated antibodies.
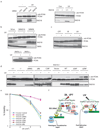
Figure 8. RAD18 participates in two distinct DNA repair pathways
(a) RAD18 is required for PCNA monoubiquitination following UV damage. Control cells, HeLa cells depleted of endogenous RAD18 (left panel) or RAD18 deficient MEF cells (right panel) were treated with 60 J/m2 of UV irradiation or left untreated. Chromatin fractions were isolated as described in the Experimental Procedures and immunoblotted with indicated antibodies. (b) RNF8 is not required for UV-induced PCNA monoubiquitination. Experiments were carried out similar to that described in (a). (c) Monoubiquitination of PCNA in cells following UV light, X-ray or CPT treatment. HeLa cells were irradiated with UV light (60 J/m2), X-rays (10 Gy) or treated with CPT (50 nM) and were allowed to recover for four, six and sixteen hours, respectively. Chromatin fractions were isolated as described in Experimental Procedures and immunoblotted with indicated antibodies. (d) Both the SAP domain and E3 ligase activity of RAD18 are required for PCNA monoubiquitination. UV-induced PCNA monoubiquitination were analyzed by Western blotting in control or RAD18−/− cells reconstituted with wild-type or various deletion/point mutants of RAD18. The experiments were carried out similar to that described in (a). (e) The zinc finger domain of RAD18 is not required for cell survival after UV treatment. Clonogenic assays were performed according to the Experimental Procedures. Results were averages of three independent experiments and presented as mean±SEM. (f) A model for RAD18 function in mediating PRR and HR repair pathways. See “Discussion” for details.
Similar articles
- Human RAD18 interacts with ubiquitylated chromatin components and facilitates RAD9 recruitment to DNA double strand breaks.
Inagaki A, Sleddens-Linkels E, van Cappellen WA, Hibbert RG, Sixma TK, Hoeijmakers JH, Grootegoed JA, Baarends WM. Inagaki A, et al. PLoS One. 2011;6(8):e23155. doi: 10.1371/journal.pone.0023155. Epub 2011 Aug 17. PLoS One. 2011. PMID: 21858012 Free PMC article. - A small ubiquitin binding domain inhibits ubiquitin-dependent protein recruitment to DNA repair foci.
Helchowski CM, Skow LF, Roberts KH, Chute CL, Canman CE. Helchowski CM, et al. Cell Cycle. 2013 Dec 15;12(24):3749-58. doi: 10.4161/cc.26640. Epub 2013 Oct 3. Cell Cycle. 2013. PMID: 24107634 Free PMC article. - Rad18 E3 ubiquitin ligase activity mediates Fanconi anemia pathway activation and cell survival following DNA Topoisomerase 1 inhibition.
Palle K, Vaziri C. Palle K, et al. Cell Cycle. 2011 May 15;10(10):1625-38. doi: 10.4161/cc.10.10.15617. Epub 2011 May 15. Cell Cycle. 2011. PMID: 21478670 Free PMC article. - The role of RAD6 in recombinational repair, checkpoints and meiosis via histone modification.
Game JC, Chernikova SB. Game JC, et al. DNA Repair (Amst). 2009 Apr 5;8(4):470-82. doi: 10.1016/j.dnarep.2009.01.007. Epub 2009 Feb 18. DNA Repair (Amst). 2009. PMID: 19230796 Review. - The ubiquitous role of ubiquitin in the DNA damage response.
Al-Hakim A, Escribano-Diaz C, Landry MC, O'Donnell L, Panier S, Szilard RK, Durocher D. Al-Hakim A, et al. DNA Repair (Amst). 2010 Dec 10;9(12):1229-40. doi: 10.1016/j.dnarep.2010.09.011. Epub 2010 Nov 4. DNA Repair (Amst). 2010. PMID: 21056014 Free PMC article. Review.
Cited by
- Rap80 protein recruitment to DNA double-strand breaks requires binding to both small ubiquitin-like modifier (SUMO) and ubiquitin conjugates.
Hu X, Paul A, Wang B. Hu X, et al. J Biol Chem. 2012 Jul 20;287(30):25510-9. doi: 10.1074/jbc.M112.374116. Epub 2012 Jun 11. J Biol Chem. 2012. PMID: 22689573 Free PMC article. - Rad18-dependent SUMOylation of human specialized DNA polymerase eta is required to prevent under-replicated DNA.
Despras E, Sittewelle M, Pouvelle C, Delrieu N, Cordonnier AM, Kannouche PL. Despras E, et al. Nat Commun. 2016 Nov 4;7:13326. doi: 10.1038/ncomms13326. Nat Commun. 2016. PMID: 27811911 Free PMC article. - Class switching and meiotic defects in mice lacking the E3 ubiquitin ligase RNF8.
Santos MA, Huen MS, Jankovic M, Chen HT, López-Contreras AJ, Klein IA, Wong N, Barbancho JL, Fernandez-Capetillo O, Nussenzweig MC, Chen J, Nussenzweig A. Santos MA, et al. J Exp Med. 2010 May 10;207(5):973-81. doi: 10.1084/jem.20092308. Epub 2010 Apr 12. J Exp Med. 2010. PMID: 20385748 Free PMC article. - The DNA Double-Strand Break Repair in Glioma: Molecular Players and Therapeutic Strategies.
Maksoud S. Maksoud S. Mol Neurobiol. 2022 Sep;59(9):5326-5365. doi: 10.1007/s12035-022-02915-2. Epub 2022 Jun 13. Mol Neurobiol. 2022. PMID: 35696013 Review. - RNF8 promotes efficient DSB repair by inhibiting the pro-apoptotic activity of p53 through regulating the function of Tip60.
Chen H, Shan J, Liu J, Feng Y, Ke Y, Qi W, Liu W, Zeng X. Chen H, et al. Cell Prolif. 2020 Mar;53(3):e12780. doi: 10.1111/cpr.12780. Epub 2020 Feb 7. Cell Prolif. 2020. PMID: 32031738 Free PMC article.
References
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. - PubMed
- Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. - PubMed
- Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. - PubMed
- Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. - PubMed
- Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous