Neuroprotective effects of estrogens following ischemic stroke - PubMed (original) (raw)
Review
Neuroprotective effects of estrogens following ischemic stroke
Shotaro Suzuki et al. Front Neuroendocrinol. 2009 Jul.
Abstract
Our laboratory has investigated whether and how 17beta-estradiol (E(2)) protects the brain against neurodegeneration associated with cerebrovascular stroke. We have discovered that low, physiological concentrations of E(2), which are strikingly similar to low-basal circulating levels found in cycling mice, dramatically protect the brain against stroke injury, and consequently revealed multiple signaling pathways and key genes that mediate protective action of E(2). Here we will review the discoveries comprising our current understanding of neuroprotective actions of estrogens against ischemic stroke. These findings may carry far reaching implications for improving the quality of life in aging populations.
Figures
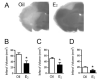
Fig. 1
Low, physiological levels of E2 exert neuroprotection. E2 protects against neuronal death in a stroke model in which the middle cerebral artery is permanently occluded. A: C57BL/6 J mice were ovariectomized and immediately implanted with capsules containing either oil or E2 for 1 week. Subsequently, animals underwent experimental ischemia by middle cerebral artery occlusion and were killed 24 h after the onset of injury. Infarct volumes were measured using TTC staining in oil- or E2-treated mice. B–D: E2 treatment significantly reduced the total infarct volume (B, P < 0.02) and the extent of injury in both the cortex (C, P < 0.05) and striatum (D, P < 0.03). Data represent the mean ± SEM of 8–11 animals per group. Figure reprinted with permission from [85].
Fig. 2
E2 attenuates markers of apoptosis. A, Photographs showing TUNEL-positive cells in the ischemic cortex from oil- and E2-treated animals at 4, 8, and 24 h after stroke injury. B, E2 significantly attenuates the number of TUNEL-positive cells during early (#P < 0.05) and late (*P < 0.05) phases of ischemic injury compared to oil-treated animals. Data represent the mean ± SEM of 8–10 animals per group. Figure reprinted with permission from [68].
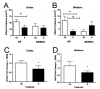
Fig. 3
E2 and iNOS display complementary neuroprotective interactions during MCAO. A, B: E2 reduces infarct volume in the cortex and striatum of WT mice ( *, P < 0.05), but does not further suppress infarct size in iNOS−/− mice. iNOS−/− oil-treated mice were also protected during stroke compared to WT oil-treated mice (#, P < 0.05); n = 8–14 mice/treatment/genotype. C, D: NOS2 gene expression was significantly higher in WT oil-treated mice than in WT E-treated mice in the cortex and striatum (*P < 0.05). NOS2 gene expression was measured in 1-mm tissue micropunches adjacent to the tissue infarct in cortex and striatum of WT mice using qRT-PCR. n = 4–5 mouse cortex or striatum samples/hormone treatment. All values represent means ± SEM. Figure reprinted with permission from [9].
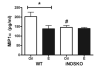
Fig. 4
E2 suppresses peripheral cytokines following MCAO. MIP1α /CCL3 was significantly suppressed by E2-treatment after MCAO injury in WT mice (*, P < 0.05), while E2 provided no further suppression in iNOS−/− mice compared to oil-treated controls. As with infarct volume, an effect of genotype was observed in iNOS−/− oil-treated mice which were also protected during stroke compared to WT oil-treated mice (#, P < 0.05, n = 8–12 mice/genotype/treatment). Values represent mean ± SEM.
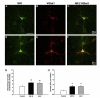
Fig. 5
E2 increases synaptic density and size. A–F, Double-label ICC for NR1 (green) and vGlut1 (red); yellow in the superimposed images indicates colocalization. G–H, E2 treatment significantly increased the density of colocalized NR1 and vGlut1 clusters and cluster size (48 h: P < 0.0005; 6 d: P < 0.003; Student’s t test). All values are mean ± SEM. Scale bars = 40 μm. Figure reprinted with permission from [37].
Fig. 6
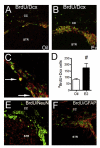
E2 significantly increases the number of BrdU+/Dcx+ newborn neurons. A, B: Confocal micrographs of BrdU+ cells (green) double-labeled with early neuronal marker doublecortin (Dcx, red) in the ipsilateral SVZ of oil- (A) vs. E2- (B) treated mice at 96 h after MCAO injury. C: Higher magnification of panel B to demonstrate colocalization of BrdU+/Dcx+. Arrows indicate representative double-labeled cells. D: E2 significantly increased the number of BrdU+/Dcx+ newborn neurons (*P = 0.0008, n = 6–7). E, F: BrdU+ cells (green) did not co-label with markers for mature neuron (NeuN, red; E) or astrocyte (GFAP, red; F). CC, corpus callosum; STR, striatum. Values represent mean ± SEM. Figure reprinted with permission from [85].
Fig. 7
E2 attenuates ischemia-induced neuroinflammation. A, B: photomicrographs of mouse brain sections stained with a marker for activated microglia (lectin). Mice were ovariectomized and treated with oil or E2 for 1 week prior to MCAO-induced ischemic injury, and collected at 24 h after injury. C: Ischemic injury increased the expression of IL-6 (*, P < 0.0001; oil-treated mice; *, P < 0.005; E2-treated mice) on the injured (ipsilateral) side of the brain compared to the contralateral side. E2 treatment attenuated ischemia-induced production of IL-6 (#P = 0.0271) on the ipsilateral side of the ischemic brain (n = 5–6 per experimental group). All values represent mean ± SEM. Figure reprinted with permission from [84].
Fig. 8
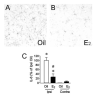
E2 facilitates migration of newborn neurons. A, B: Confocal photomicrographs of Dcx+ cells migrating toward the ischemic boundary on the ipsilateral hemisphere from oil- (A) and E2- (B) treated mice at 2 weeks after the onset of MCAO-induced injury.
Similar articles
- Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions.
Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Suzuki S, et al. Proc Natl Acad Sci U S A. 2007 Apr 3;104(14):6013-8. doi: 10.1073/pnas.0610394104. Epub 2007 Mar 26. Proc Natl Acad Sci U S A. 2007. PMID: 17389368 Free PMC article. - Estrogen, neuroprotection and neurogenesis after ischemic stroke.
Shao B, Cheng Y, Jin K. Shao B, et al. Curr Drug Targets. 2012 Feb;13(2):188-98. doi: 10.2174/138945012799201702. Curr Drug Targets. 2012. PMID: 22204318 Review. - Molecular mechanisms mediating the neuroprotective role of the selective estrogen receptor modulator, bazedoxifene, in acute ischemic stroke: A comparative study with 17β-estradiol.
Jover-Mengual T, Castelló-Ruiz M, Burguete MC, Jorques M, López-Morales MA, Aliena-Valero A, Jurado-Rodríguez A, Pérez S, Centeno JM, Miranda FJ, Alborch E, Torregrosa G, Salom JB. Jover-Mengual T, et al. J Steroid Biochem Mol Biol. 2017 Jul;171:296-304. doi: 10.1016/j.jsbmb.2017.05.001. Epub 2017 May 4. J Steroid Biochem Mol Biol. 2017. PMID: 28479229 - Mechanisms of estrogens' dose-dependent neuroprotective and neurodamaging effects in experimental models of cerebral ischemia.
Strom JO, Theodorsson A, Theodorsson E. Strom JO, et al. Int J Mol Sci. 2011;12(3):1533-62. doi: 10.3390/ijms12031533. Epub 2011 Feb 25. Int J Mol Sci. 2011. PMID: 21673906 Free PMC article. Review. - Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice.
Wang HW, Liou KT, Wang YH, Lu CK, Lin YL, Lee IJ, Huang ST, Tsai YH, Cheng YC, Lin HJ, Shen YC. Wang HW, et al. J Ethnopharmacol. 2011 Oct 31;138(1):22-33. doi: 10.1016/j.jep.2011.06.033. Epub 2011 Jul 8. J Ethnopharmacol. 2011. PMID: 21784143
Cited by
- Effect of dexmedetomidine on ncRNA and mRNA profiles of cerebral ischemia-reperfusion injury in transient middle cerebral artery occlusion rats model.
Zhang ZZ, Nasir A, Li D, Khan S, Bai Q, Yuan F. Zhang ZZ, et al. Front Pharmacol. 2024 Aug 7;15:1437445. doi: 10.3389/fphar.2024.1437445. eCollection 2024. Front Pharmacol. 2024. PMID: 39170713 Free PMC article. - Altered brain morphology and functional connectivity in postmenopausal women: automatic segmentation of whole-brain and thalamic subnuclei and resting-state fMRI.
Kim GW, Park K, Kim YH, Jeong GW. Kim GW, et al. Aging (Albany NY). 2024 Mar 23;16(6):4965-4979. doi: 10.18632/aging.205662. Epub 2024 Mar 23. Aging (Albany NY). 2024. PMID: 38526330 Free PMC article. - Out of the core: the impact of focal ischemia in regions beyond the penumbra.
Koukalova L, Chmelova M, Amlerova Z, Vargova L. Koukalova L, et al. Front Cell Neurosci. 2024 Mar 5;18:1336886. doi: 10.3389/fncel.2024.1336886. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38504666 Free PMC article. Review. - Brain-derived neuerotrophic factor and related mechanisms that mediate and influence progesterone-induced neuroprotection.
Singh M, Krishnamoorthy VR, Kim S, Khurana S, LaPorte HM. Singh M, et al. Front Endocrinol (Lausanne). 2024 Feb 26;15:1286066. doi: 10.3389/fendo.2024.1286066. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38469139 Free PMC article. Review.
References
- Acalovschi D, Wiest T, Hartmann M, Farahmi M, Mansmann U, Auffarth GU, Grau AJ, Green FR, Grond-Ginsbach C, Schwaniger M. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34:1864–1870. - PubMed
- Alvarez RJ, Gips SJ, Moldovan N, Wilhide CC, Milliken EE, Hoang AT, Hruban RH, Silverman HS, Dang CV, Goldschmidt-Clermont PJ. 17[beta]-Estradiol inhibits apoptosis of endothelial cells. Biochem. Biophys. Res. Commun. 1997;237:372–381. - PubMed
- Arvidsson A, Collin T, Krik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. - PubMed
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145:5021–5032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG02224/AG/NIA NIH HHS/United States
- F32 AG027614/AG/NIA NIH HHS/United States
- P01 AG017164/AG/NIA NIH HHS/United States
- AG17164/AG/NIA NIH HHS/United States
- AG 27614/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous