Predicting and controlling the reactivity of immune cell populations against cancer - PubMed (original) (raw)
doi: 10.1038/msb.2009.15. Epub 2009 Apr 28.
Eran Eden, Martin Akerman, Roy Noy, Ron Wolchinsky, Orit Izhaki, Ester Schallmach, Adva Kubi, Naama Zabari, Jacob Schachter, Uri Alon, Yael Mandel-Gutfreund, Michal J Besser, Yoram Reiter
Affiliations
- PMID: 19401677
- PMCID: PMC2683719
- DOI: 10.1038/msb.2009.15
Predicting and controlling the reactivity of immune cell populations against cancer
Kfir Oved et al. Mol Syst Biol. 2009.
Abstract
Heterogeneous cell populations form an interconnected network that determine their collective output. One example of such a heterogeneous immune population is tumor-infiltrating lymphocytes (TILs), whose output can be measured in terms of its reactivity against tumors. While the degree of reactivity varies considerably between different TILs, ranging from null to a potent response, the underlying network that governs the reactivity is poorly understood. Here, we asked whether one can predict and even control this reactivity. To address this we measured the subpopulation compositions of 91 TILs surgically removed from 27 metastatic melanoma patients. Despite the large number of subpopulations compositions, we were able to computationally extract a simple set of subpopulation-based rules that accurately predict the degree of reactivity. This raised the conjecture of whether one could control reactivity of TILs by manipulating their subpopulation composition. Remarkably, by rationally enriching and depleting selected subsets of subpopulations, we were able to restore anti-tumor reactivity to nonreactive TILs. Altogether, this work describes a general framework for predicting and controlling the output of a cell mixture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
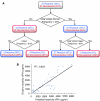
A schematic workflow of TIL characterization, analysis, and reactivity control. (A) TILs were extracted from surgically removed tumor mass originating from metastatic melanoma patients. Each TIL was characterized by functional evaluation of IFN-γ secretion levels followed by subpopulation fraction measurements using flow cytometry. This information was combined into a multiparametric model for prediction and rule-based description of TIL reactivity. Following this analysis, specific subpopulations were rationally selected for enrichment and depletion thus enabling control of TIL reactivity against melanoma. (B) Different cell surface receptors define specific T-cell subpopulations with distinct functional states. Some of these receptors are mutually exclusive (e.g. a mature T-cell will show either a CD8 or a CD4), whereas other receptors may appear simultaneously on the same cell.
Figure 2
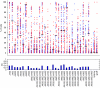
Individual subpopulations are partially predictive of TIL reactivity. For each subpopulation, blue and red dots indicate 39 reactive and 52 nonreactive TILs. The _y_-axis is the percentage of cells that belong to a specific subpopulation. The black horizontal bars indicate the optimal cutoff for classifying reactive and nonreactive TILs. The MCC classification accuracy of each subpopulation is shown at the bottom.
Figure 3
Reactive and nonreactive TILs exhibit distinct subpopulation signatures. Columns and rows correspond to TILs and subpopulations, respectively. Colors indicate the fraction of cells belonging to each subpopulation in each TIL. Unsupervised clustering was used on the rows and columns (see Materials and methods). The red and blue arrows represent nonreactive and reactive TILs, respectively. Two main clusters emerge characterized by CD4+ and CD8+ overabundant subpopulations. Interestingly, although the clustering procedure did not take into account TIL reactivity, the emerging clusters do separate nonreactive from reactive TILs (P<10−3).
Figure 4
Simple rules based on subpopulation frequencies can predict TIL reactivity. (A) A decision tree algorithm was used to generate a simple set of four rules for classifying TIL functionality (see Materials and methods). Each rule is a path from the tree root (top) to one of the leaves (bottom). (B) IFN-γ levels of reactive TILs can be described as a function of two subpopulation fractions with positive and negative weights. Each dot is a reactive TIL. The _y_-axis is the empirical IFN-γ measurements and the _x_-axis is the theoretical IFN-γ levels calculated using the following model:  Overall, IFN-γ levels of reactive TILs can be described to a large extent as a balance between two opposing subpopulations with positive and negative effects.
Overall, IFN-γ levels of reactive TILs can be described to a large extent as a balance between two opposing subpopulations with positive and negative effects.
Figure 5
Rational subpopulation manipulation can change TIL anti-tumor reactivity and is accompanied by a shift in subpopulation signature. (A) IFN-γ levels of 12 TILs before (red bars) and after (blue bars) rational subpopulation depletion and enrichment are compared. Nine of the original nonreactive TILs show significant increase in IFN-γ levels with up to 106-fold increase observed for TIL #2. Incubation of TILs in the control experiments with culture media or unrelated melanoma (white bars) indicates that the increase in IFN-γ secretion does not occur spontaneously and is tumor (HLA) specific. (B) The shift in reactivity can be explained in terms of a shift in subpopulation signature. The subpopulation fractions of 10 TILs before and after subpopulation manipulation are shown. The rows and columns correspond to different subpopulations and TILs, respectively. Two ways unsupervised clustering was performed on the rows and columns. The 10 nonreactive TILs prior to the manipulation are designated by a red color and the letter ‘P.' Ten TILs after manipulation are designated by the letter ‘A' with blue and yellow corresponding to reactive and nonreactive, respectively. Eight of the nonreactive TILs before manipulation became reactive, seven of which also showed a shift from a nonreactive subpopulation signature to a reactive one as indicated by the blue arrows going from right to left. The two TILs that remained nonreactive after manipulation exhibited either a minor change or a negative change in subpopulation signature as indicated by the red arrows. (C) The transformation of a nonreactive TIL to a reactive one can be described as a path between two points in the subpopulation space. In order to visualize the TILs positions in the multidimensional subpopulation space, we applied PCA, which is a method for dimensionality reduction (see Materials and methods). This enabled us a simple 2D visualization of the different TILs. The x and _y-_axes are the principal components capturing 49 and 24% of the total variance in the data. The _x_-axis captures a shift from CD8+ and CD28− enriched subpopulation to CD4+ and CD28+ subpopulations, whereas the _y_-axis reflects a combination of additional subpopulations (see Supplementary Figure S4 for subpopulations coefficients defining each of the principal components). The figure shows a subspace region that is overpopulated with reactive TILs. The change in reactivity can be visualized as a path from a nonreactive TIL to a TIL that resides in the reactive subspace (e.g. see dotted arrow).
Similar articles
- Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone?
Badalamenti G, Fanale D, Incorvaia L, Barraco N, Listì A, Maragliano R, Vincenzi B, Calò V, Iovanna JL, Bazan V, Russo A. Badalamenti G, et al. Cell Immunol. 2019 Sep;343:103753. doi: 10.1016/j.cellimm.2018.01.013. Epub 2018 Feb 1. Cell Immunol. 2019. PMID: 29395859 Review. - Variation of tumor-infiltrating lymphocytes in human cancers: controversy on clinical significance.
Sasada T, Suekane S. Sasada T, et al. Immunotherapy. 2011 Oct;3(10):1235-51. doi: 10.2217/imt.11.106. Immunotherapy. 2011. PMID: 21995574 Review. - Current concepts of tumor-infiltrating lymphocytes in human malignancies.
Chiou SH, Sheu BC, Chang WC, Huang SC, Hong-Nerng H. Chiou SH, et al. J Reprod Immunol. 2005 Oct;67(1-2):35-50. doi: 10.1016/j.jri.2005.06.002. Epub 2005 Aug 18. J Reprod Immunol. 2005. PMID: 16111767 Review. - Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients.
Ogiya R, Niikura N, Kumaki N, Bianchini G, Kitano S, Iwamoto T, Hayashi N, Yokoyama K, Oshitanai R, Terao M, Morioka T, Tsuda B, Okamura T, Saito Y, Suzuki Y, Tokuda Y. Ogiya R, et al. Cancer Sci. 2016 Dec;107(12):1730-1735. doi: 10.1111/cas.13101. Cancer Sci. 2016. PMID: 27727484 Free PMC article. - T cells isolated from patients with checkpoint inhibitor-resistant melanoma are functional and can mediate tumor regression.
Andersen R, Borch TH, Draghi A, Gokuldass A, Rana MAH, Pedersen M, Nielsen M, Kongsted P, Kjeldsen JW, Westergaard MCW, Radic HD, Chamberlain CA, Hölmich LR, Hendel HW, Larsen MS, Met Ö, Svane IM, Donia M. Andersen R, et al. Ann Oncol. 2018 Jul 1;29(7):1575-1581. doi: 10.1093/annonc/mdy139. Ann Oncol. 2018. PMID: 29688262 Clinical Trial.
Cited by
- Towards a better cancer precision medicine: systems biology meets immunotherapy.
Bhinder B, Elemento O. Bhinder B, et al. Curr Opin Syst Biol. 2017 Apr;2:67-73. doi: 10.1016/j.coisb.2017.01.006. Epub 2017 Mar 8. Curr Opin Syst Biol. 2017. PMID: 28989987 Free PMC article. - Cancer systems biology: embracing complexity to develop better anticancer therapeutic strategies.
Du W, Elemento O. Du W, et al. Oncogene. 2015 Jun;34(25):3215-25. doi: 10.1038/onc.2014.291. Epub 2014 Sep 15. Oncogene. 2015. PMID: 25220419 Review. - Joint modeling and registration of cell populations in cohorts of high-dimensional flow cytometric data.
Pyne S, Lee SX, Wang K, Irish J, Tamayo P, Nazaire MD, Duong T, Ng SK, Hafler D, Levy R, Nolan GP, Mesirov J, McLachlan GJ. Pyne S, et al. PLoS One. 2014 Jul 1;9(7):e100334. doi: 10.1371/journal.pone.0100334. eCollection 2014. PLoS One. 2014. PMID: 24983991 Free PMC article. - Biomarkers in the age of omics: time for a systems biology approach.
Abu-Asab MS, Chaouchi M, Alesci S, Galli S, Laassri M, Cheema AK, Atouf F, VanMeter J, Amri H. Abu-Asab MS, et al. OMICS. 2011 Mar;15(3):105-12. doi: 10.1089/omi.2010.0023. Epub 2011 Feb 14. OMICS. 2011. PMID: 21319991 Free PMC article. - Focus on adoptive T cell transfer trials in melanoma.
Hershkovitz L, Schachter J, Treves AJ, Besser MJ. Hershkovitz L, et al. Clin Dev Immunol. 2010;2010:260267. doi: 10.1155/2010/260267. Epub 2010 Dec 26. Clin Dev Immunol. 2010. PMID: 21234353 Free PMC article. Review.
References
- Alegre ML, Frauwirth KA, Thompson CB (2001) T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol 1: 220–228 - PubMed
- Benoist C, Germain RN, Mathis D (2006) A plaidoyer for ‘systems immunology'. Immunol Rev 210: 229–234 - PubMed
- Breiman L (1993) Classification and Regression Trees. Boca Raton: Chapman & Hall
- Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S (2004) Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer 91: 1711–1717 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources