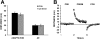Motor decline in clinically presymptomatic spinocerebellar ataxia type 2 gene carriers - PubMed (original) (raw)
Motor decline in clinically presymptomatic spinocerebellar ataxia type 2 gene carriers
Luis Velázquez-Perez et al. PLoS One. 2009.
Abstract
Background: Motor deficits are a critical component of the clinical characteristics of patients with spinocerebellar ataxia type 2. However, there is no current information on the preclinical manifestation of those motor deficits in presymptomatic gene carriers. To further understand and characterize the onset of the clinical manifestation in this disease, we tested presymptomatic spinocerebellar ataxia type 2 gene carriers, and volunteers, in a task that evaluates their motor performance and their motor learning capabilities.
Methods and findings: 28 presymptomatic spinocerebellar ataxia type 2 gene carriers and an equal number of control volunteers matched for age and gender participated in the study. Both groups were tested in a prism adaptation task known to be sensible to both motor performance and visuomotor learning deficits. Our results clearly show that although motor learning capabilities are intact, motor performance deficits are present even years before the clinical manifestation of the disease start.
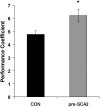
Conclusions: The results show a clear deficit in motor performance that can be detected years before the clinical onset of the disease. This motor performance deficit appears before any motor learning or clinical manifestations of the disease. These observations identify the performance coefficient as an objective and quantitative physiological biomarker that could be useful to assess the efficiency of different therapeutic agents.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Motor performance coefficient of the control (black) and the pre-SCA2 (grey) groups.
Error bars are SEM. * = p<0.01.
Figure 2. Adaptation, aftereffect, and trial-by-trial distance to target.
(A) Adaptation (left) and aftereffect (AE) (right) distance measures for the control (black) and the pre-SCA2 (grey) groups. (B) Trial-by-trial distance to target in PRE, PRISM, and POS conditions for the control (black) and the pre-SCA2 (grey) groups. Error bars are SEM. Note the lack of differences between groups in A and B.
Similar articles
- Frequency of SCA1, SCA2, SCA3/MJD, SCA6, SCA7, and DRPLA CAG trinucleotide repeat expansion in patients with hereditary spinocerebellar ataxia from Chinese kindreds.
Tang B, Liu C, Shen L, Dai H, Pan Q, Jing L, Ouyang S, Xia J. Tang B, et al. Arch Neurol. 2000 Apr;57(4):540-4. doi: 10.1001/archneur.57.4.540. Arch Neurol. 2000. PMID: 10768629 - Molecular and clinical correlation in five Indian families with spinocerebellar ataxia 12.
Srivastava AK, Choudhry S, Gopinath MS, Roy S, Tripathi M, Brahmachari SK, Jain S. Srivastava AK, et al. Ann Neurol. 2001 Dec;50(6):796-800. doi: 10.1002/ana.10048. Ann Neurol. 2001. PMID: 11761478 - Spinocerebellar ataxia type 2 in a Turkish family.
Dirik E, Yiş U, Başak N, Soydan E, Hüdaoğlu O, Ozgönül F. Dirik E, et al. J Child Neurol. 2007 Jul;22(7):891-4. doi: 10.1177/0883073807304702. J Child Neurol. 2007. PMID: 17715286 - Early onset of ataxia in a child with a pathogenic SCA8 allele.
Felling RJ, Barron TF. Felling RJ, et al. Pediatr Neurol. 2005 Aug;33(2):136-8. doi: 10.1016/j.pediatrneurol.2005.02.010. Pediatr Neurol. 2005. PMID: 16087061 Review. - [Spinocerebellar ataxia type 36 (nicknamed Asidan)].
Abe K, Ikeda Y. Abe K, et al. Brain Nerve. 2012 Aug;64(8):937-41. Brain Nerve. 2012. PMID: 22868885 Review. Japanese.
Cited by
- Tracking longitudinal thalamic volume changes during early stages of SCA1 and SCA2.
Grisoli M, Nigri A, Medina Carrion JP, Palermo S, Demichelis G, Giacosa C, Mongelli A, Fichera M, Nanetti L, Mariotti C. Grisoli M, et al. Radiol Med. 2024 Aug;129(8):1215-1223. doi: 10.1007/s11547-024-01839-2. Epub 2024 Jul 2. Radiol Med. 2024. PMID: 38954239 Free PMC article. - SCA2 predictive testing in Cuba: challenging concepts and protocol evolution.
Cruz-Mariño T, Vázquez-Mojena Y, Velázquez-Pérez L, González-Zaldívar Y, Aguilera-Rodríguez R, Velázquez-Santos M, Estupiñán-Rodríguez A, Laffita-Mesa JM, Almaguer-Mederos LE, Paneque M. Cruz-Mariño T, et al. J Community Genet. 2015 Jul;6(3):265-73. doi: 10.1007/s12687-015-0226-4. Epub 2015 Apr 19. J Community Genet. 2015. PMID: 25893506 Free PMC article. - Spinocerebellar ataxias: prospects and challenges for therapy development.
Ashizawa T, Öz G, Paulson HL. Ashizawa T, et al. Nat Rev Neurol. 2018 Oct;14(10):590-605. doi: 10.1038/s41582-018-0051-6. Nat Rev Neurol. 2018. PMID: 30131520 Free PMC article. Review. - Spinocerebellar ataxia type 2 neurodegeneration differentially affects error-based and strategic-based visuomotor learning.
Vaca-Palomares I, Díaz R, Rodríguez-Labrada R, Medrano-Montero J, Vázquez-Mojena Y, Velázquez-Pérez L, Fernandez-Ruiz J. Vaca-Palomares I, et al. Cerebellum. 2013 Dec;12(6):848-55. doi: 10.1007/s12311-013-0496-5. Cerebellum. 2013. PMID: 23754233 - Clinical, Imaging, and Laboratory Markers of Premanifest Spinocerebellar Ataxia 1, 2, 3, and 6: A Systematic Review.
Kim DH, Kim R, Lee JY, Lee KM. Kim DH, et al. J Clin Neurol. 2021 Apr;17(2):187-199. doi: 10.3988/jcn.2021.17.2.187. J Clin Neurol. 2021. PMID: 33835738 Free PMC article. Review.
References
- Velazquez-Perez L, Seifried C, Santos-Falcon N, Abele M, Ziemann U, et al. Saccade velocity is controlled by polyglutamine size in spinocerebellar ataxia 2. Ann Neurol. 2004;56:444–447. - PubMed
- Fernandez-Ruiz J, Diaz R, Hall-Haro C, Vergara P, Fiorentini A, et al. Olfactory dysfunction in hereditary ataxia and basal ganglia disorders. Neuroreport. 2003;14:1339–1341. - PubMed
- Velazquez-Perez L, Fernandez-Ruiz J, Diaz R, Gonzalez RP, Ochoa NC, et al. Spinocerebellar ataxia type 2 olfactory impairment shows a pattern similar to other major neurodegenerative diseases. J Neurol. 2006;253:1165–1169. - PubMed
- Durr A, Smadja D, Cancel G, Lezin A, Stevanin G, et al. Autosomal dominant cerebellar ataxia type I in Martinique (French West Indies). Clinical and neuropathological analysis of 53 patients from three unrelated SCA2 families. Brain. 1995;118 ( Pt 6):1573–1581. - PubMed
- Orozco G, Estrada R, Perry TL, Arana J, Fernandez R, et al. Dominantly inherited olivopontocerebellar atrophy from eastern Cuba. Clinical, neuropathological, and biochemical findings. J Neurol Sci. 1989;93:37–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources