Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes - PubMed (original) (raw)
Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes
Kanako Tamura et al. Mol Biol Cell. 2009 Jun.
Abstract
Two small GTPase Rabs, Rab32 and Rab38, have recently been proposed to regulate trafficking of melanogenic enzymes to melanosomes in mammalian epidermal melanocytes; however, the exact molecular mechanism of Rab32/38-mediated transport of melanogenic enzymes has never been clarified, because no Rab32/38-specific effector has ever been identified. In this study, we screened for a Rab32/38-specific effector by a yeast two-hybrid assay using a guanosine triphosphate (GTP)-locked Rab32/38 as bait and found that VPS9-ankyrin-repeat protein (Varp)/Ankrd27, characterized previously as a guanine nucleotide exchange factor (GEF) for Rab21, functions as a specific Rab32/38-binding protein in mouse melanocyte cell line melan-a. Deletion analysis showed that the first ankyrin-repeat (ANKR1) domain functions as a GTP-dependent Rab32/38-binding domain, but that the N-terminal VPS9 domain (i.e., Rab21-GEF domain) does not. Small interfering RNA-mediated knockdown of endogenous Varp in melan-a cells caused a dramatic reduction in Tyrp1 (tyrosinase-related protein 1) signals from melanosomes but did not cause any reduction in Pmel17 signals. Furthermore, expression of the ANKR1 domain in melan-a cells also caused a dramatic reduction of Tyrp1 signals, whereas the VPS9 domain had no effect. Based on these findings, we propose that Varp functions as the Rab32/38 effector that controls trafficking of Tyrp1 in melanocytes.
Figures
Figure 1.
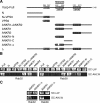
Rab binding specificity of Varp as revealed by yeast two-hybrid panels. (A) Specific interaction of Varp-Full with the GTP-fixed form of Rab32 and Rab38, and of Cep63 with the GTP-fixed form of Rab32. Yeast cells containing pGBD plasmid expressing GTP-locked Rab protein (positions indicated in the left panels) and pAct2 plasmid expressing Varp (or Cep63) protein were streaked on SC-AHLW and incubated at 30°C. Positive patches are boxed. (B) Specific interaction of Varp-Full and Varp-N+VPS9 with the nucleotide-free form of Rab21. Yeast cells containing pGBD plasmid expressing Varp protein and pGAD plasmid expressing nucleotide-free Rab (positions indicated in the left panels) were streaked on SC-AHLW and incubated at 30°C. Positive patches are boxed. (C) Schematic representation of Varp and its truncated mutant Varp-N+VPS9 used in A and B. Varp contains an N-terminal VPS9 domain and C-terminal tandem ankyrin repeats (named ANKR1 and ANKR2).
Figure 2.
Varp interacts with Rab32 and Rab38 in mammalian cells. (A) Varp interacts with Rab32 and Rab38, but not with Rab27A, in the presence of 0.5 mM guanosine 5′-_O_-(3-thio)triphosphate (GTPγS). T7-tagged Varp-Full and FLAG-tagged Rab27A/32/38 were expressed in COS-7 cells, and their associations were analyzed by coimmunoprecipitation assay using anti-FLAG tag antibody-conjugated agarose beads as described previously (Fukuda and Kanno, 2005). Coimmunoprecipitated T7-Varp-Full (middle) and immunoprecipitated FLAG-Rabs (bottom) were detected with HRP-conjugated anti-T7 tag antibody and HRP-conjugated anti-FLAG tag antibody, respectively. Input means 1/80 volume of the reaction mixture used for immunoprecipitation (top). (B) GTP-dependent interaction between Varp and Rab38. Binding assay was performed in the presence of 0.5 mM GTPγS (lane 1) or 1 mM GDP (lane 2) as described in A. (C) Direct interaction between Varp and Rab38. Agarose beads coupled with T7-Varp (arrow) were incubated with purified GST-Rab38 (lane 4, closed arrowhead) or GST alone (lane 3, open arrowhead), and bound proteins were detected with Coomassie Brilliant Blue R-250. Input means 1/25 volume of the reaction mixture (lanes 1 and 2). (D) Endogenous interaction between Varp and Rab32/38 in melan-a cells. The positions of the molecular mass markers (× 10−3) are shown on the left.
Figure 3.
Mapping of the Rab32/38-binding site in Varp. (A) Schematic representation of Varp and its truncated mutants used in this study. Varp contains an N-terminal VPS9 domain and C-terminal tandem ankyrin repeats (named ANKR1 and ANKR2). (B) ANKR1 functions as the Rab32/38-binding domain of Varp. (C) A Rab32/38-binding-defective mutant of Varp ANKR1 (named ANKR1-Δ) that lacks a second repeat in the ANKR1 (see (A)). Yeast cells containing pGAD plasmid expressing Varp mutants and pGBD plasmid expressing GTP-locked Rab32 (left) or Rab38 (right) were streaked on SC-LW (top) and SC-AHLW (bottom) and incubated at 30°C.
Figure 4.
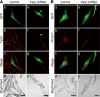
Colocalization of Varp and Rab38 in melanocytes. Melan-a cells transiently expressing GFP-Rab38 and Str-Varp (A–D), GFP-Rab38 and Str-Varp-N+VPS9 (E–H), GFP-Rab38 and Str-Varp-ANKR1 (I–L), or GFP-Rab38 and Str-Varp-Δ (M–P). Note that Varp-Full and Varp-ANKR1, but not Varp-N+VPS9 or Varp-Δ, colocalized well with Rab38 (yellow in C and K). The insets show magnified views of the boxed area. Only a few Varp- and Rab38-positive signals colocalized with melanosomes (pseudocolored in blue; arrowheads) in the perinuclear region (but see Supplemental Figure S3; colocalization between Varp and melanosomes were observed in the cell periphery). Bars, 20 μm.
Figure 5.
(A and B) Effect of the Varp shRNA on Tyrp1 staining and distribution in melan-a cells. Melan-a cells were transfected with a control vector (a–d) or Varp shRNA expression vector (e–h) together with the GFP expression vector as a transfection marker. Cells were immunostained with anti-Tyrp1 antibody (A) or anti-Pmel17 antibody (B) and visualized with Alexa Fluor 594 secondary antibody. Bright-field images show the melanosome distribution in the cells. Bars, 20 μm. Note that siRNA-mediated knockdown of Varp dramatically reduced Tyrp1-staining (arrowheads in A) but did not reduce Pmel17-staining, in melan-a cells.
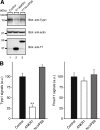
Figure 6.
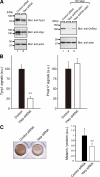
Reduced expression of Tyrp1 in Varp-deficient melanocytes. (A) Reduced expression of Tyrp1 and Varp in siVarp-treated melanocytes as revealed by immunoblotting (left panel). Cell lysates of melan-a cells treated with either siVarp or control siRNA (∼20 μg) were subjected to 10% SDS-polyacrylamide gel electrophoresis (PAGE) followed by immunoblotting with anti-Varp specific antibody (1/100 dilution), anti-Tyrp1 antibody (1/200 dilution), and anti-actin antibody (1/400 dilution). Right, efficiency and specificity of shRNA targeted against Varp were shown. Str-Varp was expressed in COS-7 cells together with Varp shRNA or a control vector. Cell lysates were subjected to 10% SDS-PAGE followed by immunoblotting with anti-DsRed antibody (1/100 dilution) and anti-actin antibody. The positions of the molecular mass markers (× 10−3) are shown on the left. (B) Reduced expression of Tyrp1 in Varp shRNA-expressing melanocytes as revealed by immunofluorescence analysis. Note that expression of Tyrp1 in Varp-deficient cells dramatically reduced, whereas expression of Pmel17 had no effect. The bars represent the means ± SE of data from three independent dishes (n > 50). **p < 0.01, Student's unpaired t test. (C) Reduced melanin content in Varp siRNA-expressing melanocytes. Melan-a cells expressing Varp siRNA (right) and control siRNA (left) were harvested, and their melanin content was assayed by measuring optical density at 490 nm. The bars represent the means ± SD of data from triplicate experiments. **p < 0.01, Student's unpaired t test.
Figure 7.
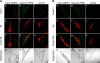
Effect of the Varp-ANKR1 domain on Tyrp1 staining and distribution in melan-a cells. Melan-a cells transiently expressing Str-Varp-ANKR1 (a–d), Str-Varp-N+VPS9 (e–h), or control Str alone (i–l) were immunostained with anti-Tyrp1 antibody (A) or anti-Pmel17 antibody (B) and visualized with Alexa Fluor 488 secondary antibody. Bright-field images show the melanosome distribution in the cells. Note that expression of Varp-ANKR1, but not of Varp-N+VPS9, caused the dramatic reduction in Tyrp1 signals (A), whereas no effect was observed for Pmel17 signals (B). Bars, 20 μm.
Figure 8.
Reduced expression of Tyrp1 in Varp-ANKR1-expressing melanocytes. (A) Reduced expression of Tyrp1 in Varp-ANKR1–expressing melanocytes as revealed by immunoblotting. Cell lysates of B16-F1 cells expressing either T7-Varp-ANKR1 or T7-Varp-N+VPS9 (∼30 μg) were subjected to 10% SDS-PAGE followed by immunoblotting with anti-Tyrp1 antibody (1/200 dilution), anti-actin antibody (1/400 dilution), and anti-T7 tag antibody (1/5000 dilution). The positions of the molecular mass markers (× 10−3) are shown on the left. (B) Reduced expression of Tyrp1 in Varp-ANKR1–expressing melanocytes as revealed by immunofluorescence analysis. Note that expression of Tyrp1 in Varp-ANKR1–expressing cells dramatically reduced, whereas expression of Pmel17 had no effect. Expression of Tyrp1 in Varp-N+VPS9–expressing cells was slightly increased in comparison with the control cells, but this was not statistically significant under our experimental conditions. The bars represent the means ± SE of data from three independent dishes (n > 50). **p < 0.01, Student's unpaired t test.
Similar articles
- Structure-function analysis of VPS9-ankyrin-repeat protein (Varp) in the trafficking of tyrosinase-related protein 1 in melanocytes.
Tamura K, Ohbayashi N, Ishibashi K, Fukuda M. Tamura K, et al. J Biol Chem. 2011 Mar 4;286(9):7507-21. doi: 10.1074/jbc.M110.191205. Epub 2010 Dec 26. J Biol Chem. 2011. PMID: 21187289 Free PMC article. - The Rab21-GEF activity of Varp, but not its Rab32/38 effector function, is required for dendrite formation in melanocytes.
Ohbayashi N, Yatsu A, Tamura K, Fukuda M. Ohbayashi N, et al. Mol Biol Cell. 2012 Feb;23(4):669-78. doi: 10.1091/mbc.E11-04-0324. Epub 2011 Dec 14. Mol Biol Cell. 2012. PMID: 22171327 Free PMC article. - RUTBC1 Functions as a GTPase-activating Protein for Rab32/38 and Regulates Melanogenic Enzyme Trafficking in Melanocytes.
Marubashi S, Shimada H, Fukuda M, Ohbayashi N. Marubashi S, et al. J Biol Chem. 2016 Jan 15;291(3):1427-40. doi: 10.1074/jbc.M115.684043. Epub 2015 Nov 30. J Biol Chem. 2016. PMID: 26620560 Free PMC article. - Multiple Roles of VARP in Endosomal Trafficking: Rabs, Retromer Components and R-SNARE VAMP7 Meet on VARP.
Fukuda M. Fukuda M. Traffic. 2016 Jul;17(7):709-19. doi: 10.1111/tra.12406. Epub 2016 May 13. Traffic. 2016. PMID: 27103185 Review. - Rab32 subfamily small GTPases: pleiotropic Rabs in endosomal trafficking.
Ohbayashi N, Fukuda M, Kanaho Y. Ohbayashi N, et al. J Biochem. 2017 Aug 1;162(2):65-71. doi: 10.1093/jb/mvx027. J Biochem. 2017. PMID: 28430987 Review.
Cited by
- Overlapping Machinery in Lysosome-Related Organelle Trafficking: A Lesson from Rare Multisystem Disorders.
Banushi B, Simpson F. Banushi B, et al. Cells. 2022 Nov 21;11(22):3702. doi: 10.3390/cells11223702. Cells. 2022. PMID: 36429129 Free PMC article. Review. - Cell type-specific Rab32 and Rab38 cooperate with the ubiquitous lysosome biogenesis machinery to synthesize specialized lysosome-related organelles.
Bultema JJ, Di Pietro SM. Bultema JJ, et al. Small GTPases. 2013 Jan-Mar;4(1):16-21. doi: 10.4161/sgtp.22349. Epub 2012 Dec 17. Small GTPases. 2013. PMID: 23247405 Free PMC article. Review. - A Comprehensive Review of Mammalian Pigmentation: Paving the Way for Innovative Hair Colour-Changing Cosmetics.
Fernandes B, Cavaco-Paulo A, Matamá T. Fernandes B, et al. Biology (Basel). 2023 Feb 11;12(2):290. doi: 10.3390/biology12020290. Biology (Basel). 2023. PMID: 36829566 Free PMC article. Review. - Lysosome-related organelles: unusual compartments become mainstream.
Marks MS, Heijnen HF, Raposo G. Marks MS, et al. Curr Opin Cell Biol. 2013 Aug;25(4):495-505. doi: 10.1016/j.ceb.2013.04.008. Epub 2013 May 29. Curr Opin Cell Biol. 2013. PMID: 23726022 Free PMC article. Review.
References
- Ando H., Watabe H., Valencia J. C., Yasumoto K., Furumura M., Funasaka Y., Oka M., Ichihashi M., Hearing V. J. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: a new aspect of ubiquitin-proteasome function. J. Biol. Chem. 2004;279:15427–15433. - PubMed
- Ando H., Kondoh H., Ichihashi M., Hearing V. J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Invest. Dermatol. 2007;127:751–761. - PubMed
- Bennett D. C., Cooper P. J., Hart I. R. A line of nontumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer. 1987;39:414–418. - PubMed
- Carney D. S., Davies B. A., Horazdovsky B. F. Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons. Trends Cell Biol. 2006;16:27–35. - PubMed
- Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc 2, identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J. Biol. Chem. 2003;278:15373–15380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous