Magnetoreception in birds: different physical processes for two types of directional responses - PubMed (original) (raw)
Magnetoreception in birds: different physical processes for two types of directional responses
Roswitha Wiltschko et al. HFSP J. 2007 May.
Abstract
Migratory orientation in birds involves an inclination compass based on radical-pair processes. Under certain light regimes, however, "fixed-direction" responses are observed that do not undergo the seasonal change between spring and autumn typical for migratory orientation. To identify the underlying transduction mechanisms, we analyzed a fixed-direction response under a combination of 502 nm turquoise and 590 nm yellow light, with migratory orientation under 565 nm green light serving as the control. High-frequency fields, diagnostic for a radical-pair mechanism, disrupted migratory orientation without affecting fixed-direction responses. Local anaesthesia of the upper beak where magnetite is found in birds, in contrast, disrupted the fixed-direction response without affecting migratory orientation. The two types of responses are thus based on different physical principles, with the compass response based on a radical pair mechanism and the fixed-direction responses probably originating in magnetite-based receptors in the upper beak. Directional input from these receptors seems to affect the behavior only when the regular inclination compass does not work properly. Evolutionary considerations suggest that magnetite-based receptors may represent an ancient mechanism that, in birds, has been replaced by the modern inclination compass based on radical-pair processes now used for directional orientation.
Figures
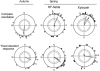
Figure 1. Orientation of European robins, Erithacus rubecula , in the local geomagnetic field; effects of a broadband high-frequency field and of Xylocain, a local anaesthetic, applied to the skin of the upper beak.
Upper diagrams: compass orientation in the migratory direction under 565 nm green light; lower diagrams: fixed-direction responses under a combination of 502 nm turquoise and 590 nm yellow light. The symbols at the periphery of the circle mark the mean headings of the test birds based on three recordings each. Open symbols: when untreated, solid symbols, with high-frequency fields added or with the local anaesthetic applied, respectively. The arrows represent the corresponding mean vectors, and the two inner circles are the 5% (dotted) and the 1% significance border of the Rayleigh test (Batschelet, 1981).
Similar articles
- Interaction of magnetite-based receptors in the beak with the visual system underlying 'fixed direction' responses in birds.
Wiltschko R, Gehring D, Denzau S, Güntürkün O, Wiltschko W. Wiltschko R, et al. Front Zool. 2010 Aug 13;7:24. doi: 10.1186/1742-9994-7-24. Front Zool. 2010. PMID: 20707905 Free PMC article. - Interactions between the visual and the magnetoreception system: different effects of bichromatic light regimes on the directional behavior of migratory birds.
Wiltschko R, Dehe L, Gehring D, Thalau P, Wiltschko W. Wiltschko R, et al. J Physiol Paris. 2013 Jan-Apr;107(1-2):137-46. doi: 10.1016/j.jphysparis.2012.03.003. Epub 2012 Apr 11. J Physiol Paris. 2013. PMID: 22504660 - Two different types of light-dependent responses to magnetic fields in birds.
Wiltschko R, Ritz T, Stapput K, Thalau P, Wiltschko W. Wiltschko R, et al. Curr Biol. 2005 Aug 23;15(16):1518-23. doi: 10.1016/j.cub.2005.07.037. Curr Biol. 2005. PMID: 16111946 - Directional orientation of birds by the magnetic field under different light conditions.
Wiltschko R, Stapput K, Thalau P, Wiltschko W. Wiltschko R, et al. J R Soc Interface. 2010 Apr 6;7 Suppl 2(Suppl 2):S163-77. doi: 10.1098/rsif.2009.0367.focus. Epub 2009 Oct 28. J R Soc Interface. 2010. PMID: 19864263 Free PMC article. Review. - Magnetic orientation and magnetoreception in birds and other animals.
Wiltschko W, Wiltschko R. Wiltschko W, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005 Aug;191(8):675-93. doi: 10.1007/s00359-005-0627-7. Epub 2005 May 11. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005. PMID: 15886990 Review.
Cited by
- Lidocaine is a nocebo treatment for trigeminally mediated magnetic orientation in birds.
Engels S, Treiber CD, Salzer MC, Michalik A, Ushakova L, Keays DA, Mouritsen H, Heyers D. Engels S, et al. J R Soc Interface. 2018 Aug;15(145):20180124. doi: 10.1098/rsif.2018.0124. J R Soc Interface. 2018. PMID: 30089685 Free PMC article. - Magnetoreception in birds: no intensity window in "fixed direction" responses.
Wiltschko W, Dehe L, Stapput K, Thalau P, Wiltschko R. Wiltschko W, et al. Naturwissenschaften. 2010 Jan;97(1):37-42. doi: 10.1007/s00114-009-0608-8. Epub 2009 Sep 17. Naturwissenschaften. 2010. PMID: 19760275 - Spontaneous magnetic alignment behaviour in free-living lizards.
Diego-Rasilla FJ, Pérez-Mellado V, Pérez-Cembranos A. Diego-Rasilla FJ, et al. Naturwissenschaften. 2017 Apr;104(3-4):13. doi: 10.1007/s00114-017-1439-7. Epub 2017 Mar 1. Naturwissenschaften. 2017. PMID: 28251303 - Can altered magnetic field affect the foraging behaviour of ants?
Pereira MC, Guimarães IC, Acosta-Avalos D, Antonialli Junior WF. Pereira MC, et al. PLoS One. 2019 Nov 25;14(11):e0225507. doi: 10.1371/journal.pone.0225507. eCollection 2019. PLoS One. 2019. PMID: 31765398 Free PMC article. - Light alters nociceptive effects of magnetic field shielding in mice: intensity and wavelength considerations.
Prato FS, Desjardins-Holmes D, Keenliside LD, McKay JC, Robertson JA, Thomas AW. Prato FS, et al. J R Soc Interface. 2009 Jan 6;6(30):17-28. doi: 10.1098/rsif.2008.0156. J R Soc Interface. 2009. PMID: 18583276 Free PMC article.
References
- Ahmad, M (2003). “Cryptochromes and flavoprotein blue-light photoreceptors.” In Handbook of Photochemistry and Photobiology, Vol. 4, pp. 159–182.
- Bailey, M J, Chong, N W, Xiong, J, and Cassone, V M (2002). “Chickens’ Cry2: molecular analysis of an avian cryptochrome in retinal and pineal photoreceptors.” FEBS Lett. FEBLAL 513, 169–174. - PubMed
- Bassart, J, Kirschvink, J L, Phillips, J B, and Borland, S C (1999). “Ferromagnetic material in the eastern red-spotted newt Notophthalmus viridescens.” J. Exp. Biol. JEBIAM 202, 3155–3160. - PubMed
LinkOut - more resources
Full Text Sources