Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1 - PubMed (original) (raw)
Comparative Study
. 2009 May;60(5):1390-405.
doi: 10.1002/art.24443.
Affiliations
- PMID: 19404941
- PMCID: PMC4879830
- DOI: 10.1002/art.24443
Comparative Study
Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1
Tomoyuki Matsumoto et al. Arthritis Rheum. 2009 May.
Abstract
Objective: The control of angiogenesis during chondrogenic differentiation is an important issue affecting the use of stem cells in cartilage repair, especially with regard to the persistence of regenerated cartilage. This study was undertaken to investigate the effect of vascular endothelial growth factor (VEGF) stimulation and the blocking of VEGF with its antagonist, soluble Flt-1 (sFlt-1), on the chondrogenesis of skeletal muscle-derived stem cells (MDSCs) in a rat model of osteoarthritis (OA).
Methods: We investigated the effect of VEGF on cartilage repair in an immunodeficiency rat model of OA after intraarticular injection of murine MDSCs expressing bone morphogenetic protein 4 (BMP-4) in combination with MDSCs expressing VEGF or sFlt-1.
Results: In vivo, a combination of sFlt-1- and BMP-4-transduced MDSCs demonstrated better repair without osteophyte formation macroscopically and histologically following OA induction, when compared with the other groups. Higher differentiation/proliferation and lower levels of chondrocyte apoptosis were also observed in sFlt-1- and BMP-4-transduced MDSCs compared with a combination of VEGF- and BMP-4-transduced MDSCs or with BMP-4-transduced MDSCs alone. In vitro experiments with mixed pellet coculture of MDSCs and OA chondrocytes revealed that BMP-4-transduced MDSCs produced the largest pellets, which had the highest gene expression of not only type II collagen and SOX9 but also type X collagen, suggesting formation of hypertrophic chondrocytes.
Conclusion: Our results demonstrate that MDSC-based therapy involving sFlt-1 and BMP-4 repairs articular cartilage in OA mainly by having a beneficial effect on chondrogenesis by the donor and host cells as well as by preventing angiogenesis, which eventually prevents cartilage resorption, resulting in persistent cartilage regeneration and repair.
Figures
Figure 1
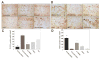
A and B, Macroscopic (A) and histologic (B) evaluation of representative joints from rats injected with muscle-derived stem cells (MDSCs) transduced with soluble Flt-1 (sFlt-1) and bone morphogenetic protein 4 (BMP-4 [B4]) (sFlt-1/BMP-4-MDSC), MDSCs transduced with vascular endothelial growth factor (VEGF) and BMP-4 (VEGF/BMF-4-MDSC), MDSCs transduced with BMP-4 alone (BMP-4-MDSC), nontransduced MDSCs (MDSC), or phosphate buffered saline (PBS) alone, 4 and 12 weeks after transplantation. Four weeks after transplantation, the sFlt-1/BMP-4–MDSC and BMP-4–MDSC groups macroscopically and histologically showed smooth joint surface with well-repaired articular cartilage and Safranin O-positive hyaline-like cartilage (red staining in B). However, the other groups showed marked arthritic progression, synovial hypertrophy, and osteophyte formation (arrows). Twelve weeks after transplantation, although the sFlt-1/BMP-4–MDSC group still showed well-repaired articular cartilage, the other groups exhibited more severe arthritis compared with 4 weeks. (Original magnification × 100.) C. Semiquantitative histologic scores for all groups, 4 and 12 weeks following transplantation. The sFlt-1/BMP-4-MDSC group had the lowest (best) scores of all groups. Bars show the mean and SEM. ** = P < 0.05 versus all other groups; * = P < 0.05 versus the VEGF/BMP-4-MDSC, MDSC, and PBS groups.
Figure 2
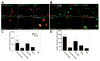
Contribution of MDSCs to cartilage regeneration and repair. A, Double immunohistochemical staining for type II collagen (Col2) and green fluorescent protein (GFP). The sFlt-1/BMP-4-MDSC and BMP-4-MDSC groups showed significantly higher levels of chondrogenic differentiation than did the other groups. B, Double immunohistochemical staining for Col2 and β_-galactosidase (β_-gal). The sFlt-1/BMP4-MDSC group showed higher levels of chondrogenic differentiation than did the VEGF/BMP-4-MDSC group. In A and B, the last panel shows a higher-magnification view of the boxed area in the first panel. Arrows show double-positive cells. Bars = 20 _μ_m. C, Numbers of GFP-positive and _β_-gal-positive cells in each group. The total chondrogenic differentiation of MDSCs was significantly greater in the sFlt-1/BMP-4-MDSC group than in the other groups. Bars show the mean and SEM. * =P< 0.05 versus the VEGF/BMP-4-MDSC and MDSC groups; †† = P < 0.05 versus all other groups; † = P < 0.05 versus the VECF/BMP-4-MDSC, MDSC, and PBS groups: # = P < 0.05 versus the VEGF/BMP-4-MDSC group. D, Total number of Col2-positive cells in each group. The sFlt-l/BMP-4-MDSC group had a significantly greater number of chondrocytes than did the other groups. Bars show the mean and SEM. ** = P < 0.05 versus all other groups; * = P < 0.05 versus the VEGF/BMP-4–MDSC, MDSC, and PBS groups. See Figure 1 for other definitions.
Figure 3
Chondrocyte apoptosis and proliferation. A, TUNEL staining in all groups 4 weeks after transplantation. The sFlt- 1/BMP-4-MDSC group had significantly fewer apoptotic cells, and the VEGF/BMP-4-MDSC group had a greater number of apoptotic cells, compared with the other groups. B, Bromodeoxyuridine (BrdU) assay in all groups 4 weeks after transplantation. The sFlt-1/BMP-4-MDSC group had a significantly greater number of proliferative cells, and the VEGF/BMP-4-MDSC group had fewer proliferative cells, compared with the other groups. In A and B, the last panel shows a higher-magnification view of the boxed area in the first panel. Bars = 50 _μ_m. C, Number of TUNEL-positive cells in each group. D, Number of BrdU-positive cells in each group. Bars in C and D show the mean and SEM. ** – P < 0.05 versus all other groups; * – P < 0.05 versus the VEGF/BMP-4-MDSC, MDSC, and PBS groups, See Figure 1 for other definitions.
Figure 4
A, Mixed pellet coculture of MDSCs and osteoarthritic (OA) chondrocytes. B, Levels of VEGF in the medium of mixed pellet coculture in each group. VEGF activity in MDSCs was blocked by sFlt-1 and enhanced by BMP-4. Bars show the mean and SEM. C, Size of pellets in mixed pellet coculture in each group. The BMP-4–MDSC plus chondrocytes group formed significantly larger pellets compared with the other groups. Bars show the mean and SEM. * = P < 0.05 versus the VEGF/BMP-4-MDSC plus chondrocytes and MDSC plus chondrocytes groups; ** = P < 0.05 versus all other groups. D, Alcian blue staining in each group. C = OA chondrocytes. E, Double immunohistochemical staining for type II collagen (Col2) and green fluorescent protein (GFP). The BMP-4–MDSC plus chondrocytes group formed a significantly greater number of chondrocytes than did the sFlt-1/BMP-4–MDSC plus chondrocytes, VEGF/BMP-4–MDSC plus chondrocytes, and MDSC plus chondrocytes groups. F, Double immunohistochemical staining for Col2 and _β_-galactosidase (_β_-gal). The sFlt-1/BMP-4–MDSC plus chondrocytes group formed a significantly greater number of chondrocytes than did the VEGF/BMP-4–MDSC plus chondrocytes group. Arrows show double-positive cells. Bars = 20 _μ_m. G, Numbers of GFP-positive and _β_-gal-positive cells in each group. Bars show the mean and SEM. ** = P < 0.05 versus all other groups; * and # = P < 0.05 versus the VEGF/BMP-4–MDSC plus chondrocytes and MDSC plus chondrocytes groups. See Figure 1 for other definitions.
Figure 5
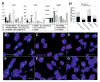
Quantitative polymerase chain reaction (PCR) and fluorescence in situ hybridization (FISH) analysis. A, Gene expression of type II collagen (Col2). SOX9, and type X collagen (Col10) in each group, as assessed by quantitative PCR analysis. Pellets from the BMP-4–MDSC plus osteoarthritic (OA) chondrocytes group showed significantly higher gene expression of Col2 and SOX9 than did the other groups; the sFlt-l/BMP-4–MDSC plus chondrocytes group had higher Col2 and SOX9 expression than did the VEGF/BMP-4–MDSC plus chondrocytes and MDSC plus chondrocytes groups. Pellets from the BMP-4–MDSC plus chondrocytes group showed significantly higher gene expression of Col10 than did the other groups; the MDSC plus chondrocytes group had higher Col10 expression than did the sFlt-l/BMP-4–MDSC plus chondrocytes and VEGF/BMP-4–MDSC plus chondrocytes groups. Bars show the mean and SEM. **, ##, and †† = P < 0.05 versus all other groups; *, #. and † = P < 0.05 versus the sFlt-1/BMP-4–MDSC plus chondrocytes and VEGF/BMP-4–MDSC plus chondrocytes groups. B–G. FISH analysis of mixed pellets of normal rat chondrocytes (B), mouse MDSCs (C), sFlt-l/BMP-4–MDSC plus chondrocytes (D), VEGF.BMP-4–MDSC plus chondrocytes (E), BMP-4–MDSC plus chondrocytes (F), and MDSC plus chondrocytes (G). demonstrating that chondrogenic differentiation of mouse MDSCs did not occur through cell fusion (complex of rat X chromosome [red] and mouse Y chromosome [green]). Bars = 20 /_μ_m. H, Quantification of chondrocytes derived from mouse MDSCs and of rat chondrocytes in each group. The sFlt-1/BMP-4–MDSC plus chondrocytes (C) and BMP-4–MDSC plus chondrocytes groups formed significantly more rat chondrocytes and mouse MDSC–derived chondrocytes than did the other groups. The total number of chondrocytes was significantly higher in the BMP-4–MDSC plus chondrocytes group. Bars show the mean and SEM. * and # = P < 0.05 versus the VEGF/BMP-4–MDSC plus chondrocytes and MDSC plus chondrocytes groups; †† = P < 005 versus all other groups; † = P < 0.05 versus the VEGF/BMP-4–MDSC plus chondrocytes and MDSC plus chondrocytes groups. See Figure 1 for other definitions.
Figure 6
A, Separated pellet coculture of MDSCs and osteoarthritic (OA) chondrocytes. B, Size of pellets in each group. OA chondrocytes in the BMP-4-MDSC plus chondrocytes group formed significantly larger pellets compared with the other groups. Bars show the mean and SEM. ** = P < 0.05 versus all other groups; * = P < 0.05 versus the VEGF/BMP-4-MDSC plus chondrocytes, MDSC plus chondrocytes, and chondrocytes alone groups. C, Alcian blue staining in each group. C = OA chondrocytes (sec Figure 1 for other definitions).
Similar articles
- Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells.
Kubo S, Cooper GM, Matsumoto T, Phillippi JA, Corsi KA, Usas A, Li G, Fu FH, Huard J. Kubo S, et al. Arthritis Rheum. 2009 Jan;60(1):155-65. doi: 10.1002/art.24153. Arthritis Rheum. 2009. PMID: 19116905 Free PMC article. - Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells.
Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, Cummins J, Fu FH, Huard J. Kuroda R, et al. Arthritis Rheum. 2006 Feb;54(2):433-42. doi: 10.1002/art.21632. Arthritis Rheum. 2006. PMID: 16447218 - The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair.
Mifune Y, Matsumoto T, Takayama K, Ota S, Li H, Meszaros LB, Usas A, Nagamune K, Gharaibeh B, Fu FH, Huard J. Mifune Y, et al. Osteoarthritis Cartilage. 2013 Jan;21(1):175-85. doi: 10.1016/j.joca.2012.09.018. Epub 2012 Oct 4. Osteoarthritis Cartilage. 2013. PMID: 23041435 - Cell-based articular cartilage repair: the link between development and regeneration.
Caldwell KL, Wang J. Caldwell KL, et al. Osteoarthritis Cartilage. 2015 Mar;23(3):351-62. doi: 10.1016/j.joca.2014.11.004. Epub 2014 Nov 11. Osteoarthritis Cartilage. 2015. PMID: 25450846 Free PMC article. Review. - Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells.
Miljkovic ND, Cooper GM, Marra KG. Miljkovic ND, et al. Osteoarthritis Cartilage. 2008 Oct;16(10):1121-30. doi: 10.1016/j.joca.2008.03.003. Epub 2008 Apr 11. Osteoarthritis Cartilage. 2008. PMID: 18406633 Review.
Cited by
- Strategies to minimize hypertrophy in cartilage engineering and regeneration.
Chen S, Fu P, Cong R, Wu H, Pei M. Chen S, et al. Genes Dis. 2015 Mar 1;2(1):76-95. doi: 10.1016/j.gendis.2014.12.003. Genes Dis. 2015. PMID: 26000333 Free PMC article. - Effects of mesenchymal stem cells on interleukin-1β-treated chondrocytes and cartilage in a rat osteoarthritic model.
Tang J, Cui W, Song F, Zhai C, Hu H, Zuo Q, Fan W. Tang J, et al. Mol Med Rep. 2015 Aug;12(2):1753-60. doi: 10.3892/mmr.2015.3645. Epub 2015 Apr 20. Mol Med Rep. 2015. PMID: 25892273 Free PMC article. - Lineage-specific multifunctional double-layer scaffold accelerates the integrated regeneration of cartilage and subchondral bone.
Ma C, Wang T, Jin X, Zhang W, Lv Q. Ma C, et al. Mater Today Bio. 2023 Sep 16;23:100800. doi: 10.1016/j.mtbio.2023.100800. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 37766897 Free PMC article. - Vascular endothelial growth factor: an essential component of angiogenesis and fracture healing.
Beamer B, Hettrich C, Lane J. Beamer B, et al. HSS J. 2010 Feb;6(1):85-94. doi: 10.1007/s11420-009-9129-4. Epub 2009 Sep 9. HSS J. 2010. PMID: 19763695 Free PMC article. - Isolation and passage of muscle-derived stem cells from the rat penile corpora cavernosa and induction of differentiation into smooth muscle cells.
Xu LJ, Xue BX, Chen D, Gao J, Yang DR, Sun CY, Cui Y, Shan YX. Xu LJ, et al. Cytotechnology. 2014 Dec;66(6):987-94. doi: 10.1007/s10616-013-9651-6. Epub 2013 Nov 19. Cytotechnology. 2014. PMID: 24242826 Free PMC article.
References
- Buckwalter JA, Stanish WD, Rosier RN, Schenck RC, Jr, Dennis DA, Coutts RD. The increasing need for nonoperative treatment of patients with osteoarthritis. Clin Orthop Relat Res. 2001:36–45. - PubMed
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. - PubMed
- Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84:571–8. - PubMed
- Visna P, Pasa L, Cizmar I, Hart R, Hoch J. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques—a randomized controlled study. Acta Chir Belg. 2004;104:709–14. - PubMed
- O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–812. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials