Induction of CCR2-dependent macrophage accumulation by oxidized phospholipids in the air-pouch model of inflammation - PubMed (original) (raw)
Induction of CCR2-dependent macrophage accumulation by oxidized phospholipids in the air-pouch model of inflammation
Alexandra Kadl et al. Arthritis Rheum. 2009 May.
Abstract
Objective: Macrophages are key players in the pathogenesis of rheumatoid synovitis as well as in atherosclerosis. To determine whether atherogenic oxidized phospholipids potentially contribute to synovial inflammation and subsequent monocyte/macrophage recruitment, we examined the effects of oxidized 1- palmitoyl-2-arachidonoyl-sn-3-glycero-phosphorylcholine (OxPAPC) on chemokine expression and leukocyte recruitment in a facsimile synovium in vivo using the murine air-pouch model.
Methods: Air pouches were raised by 2 injections of sterile air, and inflammation was induced by injecting either lipopolysaccharide (LPS) or OxPAPC into the pouch lumen. Inflammation was assessed by analysis of inflammatory gene expression using reverse transcription-polymerase chain reaction or immunohistochemical analysis, and leukocytes were quantified in the lavage fluid and in the pouch wall after staining with Giemsa or after enzymatic digestion followed by fluorescence-activated cell sorter analysis.
Results: Application of OxPAPC resulted in selective recruitment of monocyte/macrophages into the air-pouch wall, but not in the lumen. In contrast, LPS induced both monocyte and neutrophil accumulation in the pouch lumen as well as in the wall. LPS, but not OxPAPC, induced the expression of adhesion molecules E-selectin, P-selectin, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1. OxPAPC increased the expression of the CCR2 ligands monocyte chemotactic protein 1 (MCP-1), MCP-3, and MCP-5, as well as RANTES and growth-related oncogene alpha (GROalpha), while it down-regulated the expression of CCR2 on macrophages. Moreover, oxidized phospholipid-induced macrophage accumulation was abrogated in CCR2-/- mice.
Conclusion: These data demonstrate that oxidized phospholipids trigger a type of inflammatory response that leads to selective macrophage accumulation in vivo, a process relevant for the pathogenesis of chronic inflammatory rheumatic diseases.
Figures
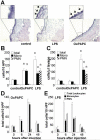
Figure 1
Induction of mononuclear cell accumulation in air-pouch tissue by injection of oxidized 1-palmitoyl-2-arachidonoyl-_sn_-glycero-3-phosphorylcholine (OxPAPC) into the air-pouch lumen. Air pouches were raised in the dorsal skin of mice and then injected with 1 ml of 0.9% saline (control) or with 1 ml of 0.9% saline containing either 250 _μ_g of OxPAPC or 50 _μ_g of lipopolysaccharide (LPS). After 24 hours, animals were euthanized, and the air-pouch tissue was analyzed. A, Representative cross-sections of the air-pouch wall show leukocytes adhering to the luminal side of the pouch membrane (arrows in insets) after injection of OxPAPC or LPS (original magnification × 4; × 20 in insets). B, Adherent cells were counted in en face preparations of air-pouch tissues from the 3 groups of mice. Cells were differentiated by morphologic characteristics. Shown are the numbers of total cells, monocytes (mono), and polymorphonuclear neutrophils (PMNs). C, Lavage was performed on air pouches in the 3 groups of mice, and the numbers of total cells, monocytes, and PMNs accumulating in the lumen were counted in the lavage fluid. D and E, Time course analyses of adherent cells in air pouches injected with OxPAPC (D) or LPS (E) were performed. Shown are the numbers of total cells, monocytes, and PMNs. Values in B–E are the mean and SEM of 4 mice per group. * = P < 0.01 versus control in B; P < 0.05 versus control in C, by analysis of variance. HPF = high-power fields. Color figure can be viewed in the online issue, which is available at
.
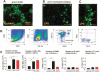
Figure 2
Selective induction of the recruitment of CD11b+/Gr-1low cells by injection of oxidized 1-palmitoyl-2-arachidonoyl-_sn_-glycero-3-phosphorylcholine (OxPAPC). Leukocytes accumulating in the air-pouch wall were analyzed in tissues collected 24 hours after injection with OxPAPC, lipopolysaccharide (LPS), or saline alone (control). A–C, Cross-sections of the air-pouch wall were stained for antibodies against F4/80 (A) or myeloperoxidase (B and C). Some myeloperoxidase-positive cells in the LPS-treated air-pouch wall are in the process of migrating into the pouch lumen (arrows). D, Tissue was weighed and enzymatically digested to obtain a single-cell suspension, which was stained with leukocyte markers and subjected to flow cytometry. The CD45+ cells were then analyzed for CD11b and Gr-1 expression. Polymorphonuclear neutrophils (PMNs) were defined as Gr-1high/CD11b+ cells and monocyte/macrophages (MN/MΦs) as Gr-1low/CD11b+. The remaining CD11b–/Gr-1– cells were defined as lymphocytes, which stained positive for CD3 (T cells) or CD19 (B cells). SSC = side scatter; FSC = forward scatter. E, Absolute numbers of leukocytes, monocyte/macrophages, PMNs, B cells, and T cells were obtained as the products of flow cytometry percentages and total cell counts in samples from the 3 experimental groups. Values are the mean and SD of 4 mice per group. * = P < 0.01 by analysis of variance. OxPL = oxidized phospholipids (i.e., OxPAPC).
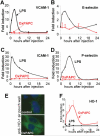
Figure 3
Induction of heme oxygenase 1 (HO-1) expression, but no increase in the expression of endothelial adhesion molecules, following injection of oxidized 1-palmitoyl-2-arachidonoyl-_sn_-glycero-3-phosphorylcholine (OxPAPC). A–D, In contrast to lipopolysaccharide (LPS; 50 _μ_g), OxPAPC (250 _μ_g) failed to induce the expression of vascular cell adhesion molecule 1 (VCAM-1) (A), E-selectin (B), intercellular adhesion molecule 1 (ICAM-1) (C), or P-selectin (D) in air-pouch tissue analyzed at the indicated time points after injection, as determined by reverse transcription–polymerase chain reaction analyses. E, Cross-sections of the air-pouch wall 24 hours after saline (control) or OxPAPC injection show HO-1 protein in the OxPAPC-injected tissue (original magnification × 40). F, Time course analysis of the expression of mRNA for HO-1. Values in A–D and F are the mean and SD of 4 mice per group. Color figure can be viewed in the online issue, which is available at
.
Figure 4
Induction of chemokine expression following injection of oxidized 1-palmitoyl-2-arachidonoyl-sn_-glycero-3-phosphorylcholine (OxPAPC). Animals were injected with either 1 ml of 0.9% saline (control) or 1 ml of 0.9% saline containing 250 μ_g of OxPAPC or 50 μ_g of lipopolysaccharide (LPS) into the air pouch and were euthanized at the indicated time points. RNA was isolated from air-pouch tissue, and the expression of monocyte chemotactic protein 1 (MCP-1)/JE, MCP-3, MCP-5, interferon-γ_–inducible 10-kd protein (IP-10), RANTES, BRAK, growth-related oncogene α (GRO_α), macrophage inflammatory protein 1_β (MIP-1_β), and MIP-1_α was analyzed by reverse transcription–polymerase chain reaction. Values are the mean and SD fold increase over controls (n = 4 mice per group). Color figure can be viewed in the online issue, which is available at
.
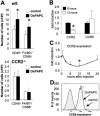
Figure 5
Requirement of CCR2 for oxidized 1-palmitoyl-2-arachidonoyl-_sn_-glycero-3-phosphorylcholine (OxPAPC)–induced monocyte recruitment. A, F4/80-positive and CD68-positive cells in the air-pouch wall from wild-type (W/T) mice (top) and from CCR2-deficient (CCR2−/−) mice (bottom) injected with saline (control) or OxPAPC were analyzed by flow cytometry. B and C, Expression of CCR2 and CCR5 in the air-pouch tissue after treatment with OxPAPC was analyzed by reverse transcription–polymerase chain reaction (B), and a time course analysis of CCR2 expression (C) was performed. D, Bone marrow–derived macrophages were stimulated with OxPAPC in vitro, and CCR2 expression was analyzed by flow cytometry. Values in A–C are the mean and SD of 4 mice per group. * = P < 0.05 versus control in A and versus 0 hours in B and C, by analysis of variance.
Similar articles
- Oxidized phospholipids trigger atherogenic inflammation in murine arteries.
Furnkranz A, Schober A, Bochkov VN, Bashtrykov P, Kronke G, Kadl A, Binder BR, Weber C, Leitinger N. Furnkranz A, et al. Arterioscler Thromb Vasc Biol. 2005 Mar;25(3):633-8. doi: 10.1161/01.ATV.0000153106.03644.a0. Epub 2004 Dec 9. Arterioscler Thromb Vasc Biol. 2005. PMID: 15591214 - Analysis of inflammatory gene induction by oxidized phospholipids in vivo by quantitative real-time RT-PCR in comparison with effects of LPS.
Kadl A, Huber J, Gruber F, Bochkov VN, Binder BR, Leitinger N. Kadl A, et al. Vascul Pharmacol. 2002 Apr;38(4):219-27. doi: 10.1016/s1537-1891(02)00172-6. Vascul Pharmacol. 2002. PMID: 12449018 - CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis.
Raghu H, Lepus CM, Wang Q, Wong HH, Lingampalli N, Oliviero F, Punzi L, Giori NJ, Goodman SB, Chu CR, Sokolove JB, Robinson WH. Raghu H, et al. Ann Rheum Dis. 2017 May;76(5):914-922. doi: 10.1136/annrheumdis-2016-210426. Epub 2016 Dec 13. Ann Rheum Dis. 2017. PMID: 27965260 Free PMC article. - [Macrophages, inflammation, adipose tissue, obesity and insulin resistance].
Bastarrachea RA, López-Alvarenga JC, Bolado-García VE, Téllez-Mendoza J, Laviada-Molina H, Comuzzie AG. Bastarrachea RA, et al. Gac Med Mex. 2007 Nov-Dec;143(6):505-12. Gac Med Mex. 2007. PMID: 18269082 Review. Spanish. - MCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expression.
Sozzani S, Introna M, Bernasconi S, Polentarutti N, Cinque P, Poli G, Sica A, Mantovani A. Sozzani S, et al. J Leukoc Biol. 1997 Jul;62(1):30-3. doi: 10.1002/jlb.62.1.30. J Leukoc Biol. 1997. PMID: 9225989 Review.
Cited by
- Peptide fragment 29-40 of amino acid sequence of monocyte chemoattractant protein-1 (MCP-1) stimulates monocyte migration in vivo and facilitates wound healing.
Arefieva TI, Sokolov VO, Pylaeva EA, Kukhtina NB, Potekhina AV, Ruleva NY, Sidorova MV, Bespalova ZhD, Azmuko AA, Krasnikova TL. Arefieva TI, et al. Dokl Biol Sci. 2012 Sep-Oct;446:327-30. doi: 10.1134/S001249661205002X. Epub 2012 Nov 6. Dokl Biol Sci. 2012. PMID: 23129286 No abstract available. - NADPH oxidases in vascular pathology.
Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. Konior A, et al. Antioxid Redox Signal. 2014 Jun 10;20(17):2794-814. doi: 10.1089/ars.2013.5607. Epub 2013 Nov 1. Antioxid Redox Signal. 2014. PMID: 24180474 Free PMC article. Review. - Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment.
Ludwig A, Otto GP, Riento K, Hams E, Fallon PG, Nichols BJ. Ludwig A, et al. J Cell Biol. 2010 Nov 15;191(4):771-81. doi: 10.1083/jcb.201005140. Epub 2010 Nov 8. J Cell Biol. 2010. PMID: 21059848 Free PMC article. - Acute inflammatory response to cobalt chromium orthopaedic wear debris in a rodent air-pouch model.
Akbar M, Fraser AR, Graham GJ, Brewer JM, Grant MH. Akbar M, et al. J R Soc Interface. 2012 Sep 7;9(74):2109-19. doi: 10.1098/rsif.2012.0006. Epub 2012 Apr 18. J R Soc Interface. 2012. PMID: 22513721 Free PMC article. - Lipoprotein(a), a Lethal Player in Calcific Aortic Valve Disease.
Hu J, Lei H, Liu L, Xu D. Hu J, et al. Front Cell Dev Biol. 2022 Jan 27;10:812368. doi: 10.3389/fcell.2022.812368. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35155427 Free PMC article. Review.
References
- Chung CP, Oeser A, Solus J, Avalos I, Gebretsadik T, Shintani A, et al. Inflammatory mechanisms affecting the lipid profile in patients with systemic lupus erythematosus. J Rheumatol. 2007;34:1849–54. - PubMed
- Dhawan SS, Quyyumi AA. Rheumatoid arthritis and cardiovascular disease. Curr Atheroscler Rep. 2008;10:128–33. - PubMed
- Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin Rheumatol. 2007;26:1228–33. - PubMed
- Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL-084422-01/HL/NHLBI NIH HHS/United States
- R01 HL084422-01/HL/NHLBI NIH HHS/United States
- R01 HL084422-02/HL/NHLBI NIH HHS/United States
- R01 HL084422-03/HL/NHLBI NIH HHS/United States
- R01 HL084422/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous