Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle - PubMed (original) (raw)
Review
Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle
Robert T Dirksen. J Physiol. 2009.
Abstract
It has long been known that skeletal muscle contraction persists in the absence of extracellular Ca(2+). Nevertheless, recent evidence indicates that multiple distinct Ca(2+) entry pathways exist in skeletal muscle: one active at negative potentials that requires store depletion (store-operated calcium entry or SOCE) and a second that is independent of store depletion and is activated by depolarization (excitation-coupled calcium entry or ECCE). This review highlights recent findings regarding the molecular identity, subcellular localization, and inter-relationship between SOCE and ECCE in skeletal muscle. The respective roles of ryanodine receptors (RyRs), dihydropyridine receptors (DHPRs), inositol-1,4,5-trisphosphate receptors (IP(3)Rs), canonical transient receptor potential channels (TRPCs), STIM1 Ca(2+) sensor proteins, and Orai1 Ca(2+) permeable channels in mediating SOCE and ECCE in skeletal muscle are discussed. Differences between SOCE and ECCE in skeletal muscle with Ca(2+) entry mechanisms in non-excitable cells are also reviewed. Finally, potential physiological roles for SOCE and ECCE in skeletal muscle development and function, as well as other currently unanswered questions and controversies in the field are also considered.
Figures
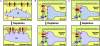
Figure 1. Proposed molecular models for SOCE in skeletal muscle
A, conformational coupling between the ryanodine receptor (RyR) and canonical transient receptor potential (TRPC) channels. B, conformational coupling between inositol-1,4,5-trisphosphate (IP3) receptors and TRPC channels. C, conformational coupling between ER/SR stromal interaction molecule 1 (STIM1) Ca2+ sensor proteins and Ca2+ permeable Orai1 channels. For clarity, only one Orai1 subunit is shown.
Figure 2. Structural features of human STIM and Orai proteins
A, structural features of human STIM proteins. EF, Ca2+ binding EF hand domain; SAM, sterile-α-motif; TM, transmembrane domain; c-c, coiled coil domain; ERM, ezrin–radixin moesin domain; S/P serine-proline-rich domain; KKK, lysine-rich domain; CAD, channel activation domain; SOAR, STIM1–Orai activation region; P/H/E, proline-histidine-glutimate-rich domain. B, structural features of human Orai proteins. P/R, proline-arginine-rich domain; TM, transmembrane domain; c-c, coiled coil domain; SCID, severe combined immunodeficiency.
Figure 3. Proposed models for activation of SOCE in T-lymphocytes and skeletal muscle
A, model for activation of SOCE in T-lymphocytes. At rest (upper), Ca2+-bound STIM1 proteins are diffusely distributed in the ER and Orai1 proteins are randomly located in the plasma membrane (shown as non-functional dimer; Penna et al. 2008). Following store depletion (lower), Ca2+ unbinding from the STIM1 EF hand results in STIM1 oligomerization and redistribution into discrete puncta under the plasma membrane. Orai1 channels are recruited into these puncta and conformational coupling between STIM1 oligomers and tetrameric Orai1 channels results in activation of SOCE. B, proposed model for rapid activation of SOCE in skeletal muscle. At rest (upper), Ca2+-bound STIM1 proteins are pre-localized to the SR terminal cisternae and Orai1 proteins are located in the t-tubule membrane. Following store depletion (lower), Ca2+ unbinding from the STIM1 EF hand results in STIM1 oligomerization, recruitment of nearby Orai1 subunit, and conformational activation of SOCE. C, alternate proposed model for ultra-rapid activation of SOCE in skeletal muscle. At rest (upper), Ca2+-bound STIM1 proteins in the SR terminal cisternae are pre-bound to inactive tetrameric Orai1 channels located in the t-tubule membrane. Following store depletion (lower), Ca2+ unbinding from the STIM1 EF hand results in an immediate conformational change in the STIM1–Orai1 interaction that rapidly activates SOCE.
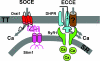
Figure 4. SOCE and ECCE muscle are determined by distinct molecular complexes within SR–sarcolemmal junctions
Left, SOCE involves ‘inside-out’ conformational coupling between SR STIM1 Ca2+ sensor proteins and tetrameric Ca2+ permeable Orai1 channels located in the t-tubule membrane. For clarity, only one Orai1 subunit is shown. Right, ECCE involves ‘outside-in’ conformational coupling between the DHPR voltage sensor and the SR Ca2+ release channel. The Ca2+ permeation pathway for ECCE involves Ca2+ flux through the L-type Ca2+ channel pore and/or an unidentified associated channel. Figure modified with permission from Lyfenko & Dirksen (2008).
Comment in
- Calsequestrin, triadin and more: the molecules that modulate calcium release in cardiac and skeletal muscle.
Ríos E, Györke S. Ríos E, et al. J Physiol. 2009 Jul 1;587(Pt 13):3069-70. doi: 10.1113/jphysiol.2009.175083. J Physiol. 2009. PMID: 19567746 Free PMC article. No abstract available. - Calcium entry in skeletal muscle.
Rosenberg PB. Rosenberg PB. J Physiol. 2009 Jul 1;587(Pt 13):3149-51. doi: 10.1113/jphysiol.2009.172585. J Physiol. 2009. PMID: 19567752 Free PMC article. Review. No abstract available.
Similar articles
- Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1.
Lyfenko AD, Dirksen RT. Lyfenko AD, et al. J Physiol. 2008 Oct 15;586(20):4815-24. doi: 10.1113/jphysiol.2008.160481. Epub 2008 Sep 4. J Physiol. 2008. PMID: 18772199 Free PMC article. - What role for store-operated Ca²⁺ entry in muscle?
Trebak M, Zhang W, Ruhle B, Henkel MM, González-Cobos JC, Motiani RK, Stolwijk JA, Newton RL, Zhang X. Trebak M, et al. Microcirculation. 2013 May;20(4):330-6. doi: 10.1111/micc.12042. Microcirculation. 2013. PMID: 23312019 Free PMC article. Review. - Conformational activation of Ca2+ entry by depolarization of skeletal myotubes.
Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, Pessah IN. Cherednichenko G, et al. Proc Natl Acad Sci U S A. 2004 Nov 2;101(44):15793-8. doi: 10.1073/pnas.0403485101. Epub 2004 Oct 25. Proc Natl Acad Sci U S A. 2004. PMID: 15505226 Free PMC article. - Conformation of ryanodine receptor-2 gates store-operated calcium entry in rat pulmonary arterial myocytes.
Lin AH, Sun H, Paudel O, Lin MJ, Sham JS. Lin AH, et al. Cardiovasc Res. 2016 Jul 1;111(1):94-104. doi: 10.1093/cvr/cvw067. Epub 2016 Mar 24. Cardiovasc Res. 2016. PMID: 27013634 Free PMC article. - Role of STIM1/ORAI1-mediated store-operated Ca2+ entry in skeletal muscle physiology and disease.
Michelucci A, García-Castañeda M, Boncompagni S, Dirksen RT. Michelucci A, et al. Cell Calcium. 2018 Dec;76:101-115. doi: 10.1016/j.ceca.2018.10.004. Epub 2018 Oct 30. Cell Calcium. 2018. PMID: 30414508 Free PMC article. Review.
Cited by
- ECC meets CEU-New focus on the backdoor for calcium ions in skeletal muscle cells.
Melzer W. Melzer W. J Gen Physiol. 2020 Oct 5;152(10):e202012679. doi: 10.1085/jgp.202012679. J Gen Physiol. 2020. PMID: 32851409 Free PMC article. - STIM1-Orai1 interaction mediated calcium influx activation contributes to cardiac contractility of insulin-resistant rats.
Durak A, Olgar Y, Genc K, Tuncay E, Akat F, Degirmenci S, Turan B. Durak A, et al. BMC Cardiovasc Disord. 2022 Apr 5;22(1):147. doi: 10.1186/s12872-022-02586-w. BMC Cardiovasc Disord. 2022. PMID: 35379188 Free PMC article. - Enhanced Ca²⁺ influx from STIM1-Orai1 induces muscle pathology in mouse models of muscular dystrophy.
Goonasekera SA, Davis J, Kwong JQ, Accornero F, Wei-LaPierre L, Sargent MA, Dirksen RT, Molkentin JD. Goonasekera SA, et al. Hum Mol Genet. 2014 Jul 15;23(14):3706-15. doi: 10.1093/hmg/ddu079. Epub 2014 Feb 20. Hum Mol Genet. 2014. PMID: 24556214 Free PMC article. - STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release.
Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. Darbellay B, et al. J Cell Biol. 2011 Jul 25;194(2):335-46. doi: 10.1083/jcb.201012157. J Cell Biol. 2011. PMID: 21788372 Free PMC article. - The cardiac alpha(1C) subunit can support excitation-triggered Ca2+ entry in dysgenic and dyspedic myotubes.
Bannister RA, Beam KG. Bannister RA, et al. Channels (Austin). 2009 Jul-Aug;3(4):268-73. doi: 10.4161/chan.3.4.9342. Epub 2009 Jul 24. Channels (Austin). 2009. PMID: 19625771 Free PMC article.
References
- Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N¢-tetracetic acid. Biochim Biophys Acta. 1972;267:605–608. - PubMed
- Balog EM, Gallant EM. Modulation of the sarcolemmal L-type current by alteration in SR Ca2+ release. Am J Physiol Cell Physiol. 1999;276:C128–C135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR044657/AR/NIAMS NIH HHS/United States
- R29 AR044657/AR/NIAMS NIH HHS/United States
- R01 AR044657-11/AR/NIAMS NIH HHS/United States
- P01 AR052354-030004/AR/NIAMS NIH HHS/United States
- P01 AR052354/AR/NIAMS NIH HHS/United States
- 5P01AR052354/AR/NIAMS NIH HHS/United States
- R01 AR044657/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous