TNF-alpha promotes an odontoblastic phenotype in dental pulp cells - PubMed (original) (raw)
TNF-alpha promotes an odontoblastic phenotype in dental pulp cells
F W G Paula-Silva et al. J Dent Res. 2009 Apr.
Abstract
Dental pulp cells can differentiate toward an odontoblastic phenotype to produce reparative dentin beneath caries lesions. However, the mechanisms involved in pulp cell differentiation under pro-inflammatory stimuli have not been well-explored. Thus, we hypothesized that the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-alpha) could be a mediator involved in dental pulp cell differentiation toward an odontoblastic phenotype. We observed that TNF-alpha-challenged pulp cells exhibited increased mineralization and early and increased expression of dentin phosphoprotein (DPP), dentin sialoprotein (DSP), dentin matrix protein-1, and osteocalcin during a phase of reduced matrix metalloproteinase (MMP) expression. We investigated whether these events were related and found that p38, a mitogen-activated protein kinase, differentially regulated MMP-1 and DSP/DPP expression and mediated mineralization upon TNF-alpha treatment. These findings indicate that TNF-alpha stimulates differentiation of dental pulp cells toward an odontoblastic phenotype via p38, while negatively regulating MMP-1 expression.
Figures
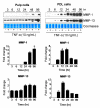
Figure 1.
Expression of mineralization-associated proteins and mineralized nodule formation after treatment with TNF-α. (A) Dental pulp and PDL cells were cultured for 6 and 24 hrs in the absence or presence of 10 ng/mL of TNF-α, and expression of DPP (87 kDa), DSP (42 kDa), DMP-1 (57 kDa), and osteocalcin (OC) (11 kDa) in cell lysates was evaluated. GAPDH (37 kDa) was used as loading control. (B) Dose-response effects of TNF-α on DPP and DSP expression were assessed at 24 hrs in dental pulp cells. (C) Extracellular levels of DPP and DSP were evaluated 6, 24, and 48 hrs in the presence or absence of TNF-α. Coomassie blue staining showed equal loading for all lanes. (D) Immunostaining for DSPP/DPP expression in dental pulp and PDL cells with or without TNF-α (10 ng/mL) treatment. The number of DSPP/DPP-positive cells was counted in 5 representative areas and expressed as a percentage of total number of cells in the field of view. Percentages of positive cells are given below the corresponding images (bar = 10 µm). (E) Mineralized nodule formation was visualized by von Kossa staining. Calcium content was quantified with the use of a calcium assay kit, and data were normalized by total protein concentration (in µg calcium/mg). Values shown above the images depict mean and standard deviation from 3 different cell donors.
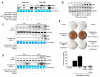
Figure 2.
Time-course effects of TNF-α on MMP-1 (55 kDa) and MMP-13 (60 kDa) secretion by dental pulp and PDL cells. Western immunoblots are representative of conditioned medium samples from cells treated with 10 ng/mL of TNF-α for 3, 6, 12, 24, 48, and 96 hrs. Coomassie blue staining shows equal loading for all lanes. Graphs represent fold-change in expression of MMP-1 and MMP-13 adjusted to baseline levels for dental pulp and PDL cells. Graphs depict mean and standard deviation from 3 different cell donors; *p < 0.05.
Figure 3.
Effects of NFκB and MAPK inhibitors on TNF-α-mediated MMP-1 and MMP-13 expression in dental pulp (A, left) and PDL (A, right) cells. Cells were pre-treated with BMS345541 (IKK phosphorylation inhibitor; 5 µM; 1.27 mg/mL), U0126 (MEK-1/2 inhibitor; 10 µM; 4.03 mg/mL), SB203580 (p38 inhibitor; 10 µM; 3.77 mg/mL), and SP600125 (JNK inhibitor; 10 µM; 2.02 mg/mL) for 1 hr and stimulated with 10 ng/mL of TNF-α for 24 hrs. Western immunoblots show basal levels of MMP-1 and MMP-13, after stimulation with the inhibitors plus TNF-α or TNF-α alone. Coomassie blue staining shows equal loading for all lanes. (B) Time-course experiments showing the effects of TNF-α on p38 (43 kDa) phosphorylation levels after treatment of dental pulp cells with TNF-α (10 ng/mL) for the indicated periods. Ratios of phosphorylated to total p38 levels were calculated by densitometric analysis and are shown above the panels. The effects of p38 MAPK inhibition on TNF-α-mediated DPP, DSP (C), and MMP-1 (D) expression in dental pulp cells were evaluated by Western immunoblotting. Cells were pre-treated with 10 µM of p38 inhibitor (SB203580) for 1 hr and then treated with TNF-α (10 ng/mL) plus p38 inhibitor (10 µM) for 6, 24, and 48 hrs. (E) Effects of p38 inhibition on mineralized nodule formation were assessed as in Fig. 1. Graphs depict mean and standard deviation from 3 different cell donors; *p < 0.05.
Figure 4.
p38 MAPK siRNA effects on TNF-α-mediated DPP/DSP and MMP-1 expression. Efficacy of p38 siRNA inhibition on total p38 protein expression was quantified after GAPDH normalization, and values are shown above the panels (A). The effects of p38 MAPK siRNA (20 or 60 pmol) on TNF-α-mediated DPP and DSP (B), and MMP-1 (C) expression in dental pulp cells were evaluated. Coomassie blue staining shows equal loading for all lanes. Graphs depict mean and standard deviation from 3 different cell donors; *p < 0.05.
Similar articles
- Hyaluronan induces odontoblastic differentiation of dental pulp stem cells via CD44.
Umemura N, Ohkoshi E, Tajima M, Kikuchi H, Katayama T, Sakagami H. Umemura N, et al. Stem Cell Res Ther. 2016 Sep 20;7(1):135. doi: 10.1186/s13287-016-0399-8. Stem Cell Res Ther. 2016. PMID: 27651223 Free PMC article. - Quaking promotes the odontoblastic differentiation of human dental pulp stem cells.
Li S, Lin C, Zhang J, Tao H, Liu H, Yuan G, Chen Z. Li S, et al. J Cell Physiol. 2018 Sep;233(9):7292-7304. doi: 10.1002/jcp.26561. Epub 2018 Apr 16. J Cell Physiol. 2018. PMID: 29663385 - Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex.
Kawashima N, Okiji T. Kawashima N, et al. Congenit Anom (Kyoto). 2016 Jul;56(4):144-53. doi: 10.1111/cga.12169. Congenit Anom (Kyoto). 2016. PMID: 27131345 Review. - Leptin in Dental Pulp and Periapical Tissues: A Narrative Review.
Martin-Gonzalez J, Segura-Egea JJ, Pérez-Pérez A, Cabanillas-Balsera D, Sánchez-Margalet V. Martin-Gonzalez J, et al. Int J Mol Sci. 2022 Feb 11;23(4):1984. doi: 10.3390/ijms23041984. Int J Mol Sci. 2022. PMID: 35216099 Free PMC article. Review.
Cited by
- The effects of mineral trioxide aggregate and second-generation autologous growth factor on pulpotomy via TNF-α and NF-kβ/p65 pathways.
Kurt A, Çıkman AŞ, Balaban E, Gümrükçü Z, Mercantepe T, Tümkaya L, Karabağ M. Kurt A, et al. BMC Oral Health. 2024 Aug 3;24(1):890. doi: 10.1186/s12903-024-04577-z. BMC Oral Health. 2024. PMID: 39097700 Free PMC article. - Analysis of the cytotoxicity and bioactivity of CeraSeal, BioRoot™ and AH Plus® sealers in pre-osteoblast lineage cells.
de Almeida-Junior LA, de Campos Chaves Lamarque G, Herrera H, Arnez MFM, Lorencetti-Silva F, Silva RAB, Silva LAB, Paula-Silva FWG. de Almeida-Junior LA, et al. BMC Oral Health. 2024 Feb 22;24(1):262. doi: 10.1186/s12903-024-04021-2. BMC Oral Health. 2024. PMID: 38389110 Free PMC article. - BDNF/TrkB Is a Crucial Regulator in the Inflammation-Mediated Odontoblastic Differentiation of Dental Pulp Stem Cells.
Kim JH, Irfan M, Hossain MA, George A, Chung S. Kim JH, et al. Cells. 2023 Jul 14;12(14):1851. doi: 10.3390/cells12141851. Cells. 2023. PMID: 37508514 Free PMC article. - Host defense peptides combined with MTA extract increase the repair in dental pulp cells: in vitro and ex vivo study.
Silva PAO, Martins DCM, de Castro Cantuária AP, de Andrade RV, Lacorte C, de Almeida JA, Aguiar LR, Corrêa JR, da Silva IGM, Franco OL, Rezende TMB. Silva PAO, et al. Sci Rep. 2023 Jun 12;13(1):9531. doi: 10.1038/s41598-023-36748-3. Sci Rep. 2023. PMID: 37308525 Free PMC article. - Characterisation of miRNA Expression in Dental Pulp Cells during Epigenetically-Driven Reparative Processes.
Kearney M, Cooper PR, Smith AJ, Duncan HF. Kearney M, et al. Int J Mol Sci. 2023 May 11;24(10):8631. doi: 10.3390/ijms24108631. Int J Mol Sci. 2023. PMID: 37239975 Free PMC article.
References
- Addison WN, Azari F, Sørensen ES, Kaartinen MT, McKee MD. (2007). Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem 282:15872-15883 - PubMed
- Bletsa A, Heyeraas KJ, Haug SR, Berggreen E. (2004). IL-1 alpha and TNF-alpha expression in rat periapical lesions and dental pulp after unilateral sympathectomy. Neuroimmunomodulation 11:376-384 - PubMed
- Butler WT, Ritchie H. (1995). The nature and functional significance of dentin extracellular matrix proteins. Int J Dev Biol 39:169-179 - PubMed
- Butler WT, Brunn JC, Qin C. (2003). Dentin extracellular matrix (ECM) proteins: comparison to bone ECM and contribution to dynamics of dentinogenesis. Connect Tissue Res 44(Suppl 1):171-178 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous