XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells - PubMed (original) (raw)
XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells
Chih-Chi Andrew Hu et al. EMBO J. 2009.
Abstract
XBP-1, a transcription factor that drives the unfolded protein response (UPR), is activated in B cells when they differentiate to plasma cells. Here, we show that in the B cells, whose capacity to secrete IgM has been eliminated, XBP-1 is induced normally on induction of differentiation, suggesting that activation of XBP-1 in B cells is a differentiation-dependent event, but not the result of a UPR caused by the abundant synthesis of secreted IgM. Without XBP-1, B cells fail to signal effectively through the B-cell receptor. The signalling defects lead to aberrant expression of the plasma cell transcription factors IRF4 and Blimp-1, and altered levels of activation-induced cytidine deaminase and sphingosine-1-phosphate receptor. Using XBP-1-deficient/Blimp-1-GFP transgenic mice, we find that XBP-1-deficient B cells form antibody-secreting plasmablasts in response to initial immunization; however, these plasmablasts respond ineffectively to CXCL12. They fail to colonize the bone marrow and do not sustain antibody production. These findings define the role of XBP-1 in normal plasma cell development and have implications for management of B-cell malignancies.
Figures
Figure 1
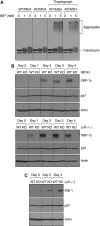
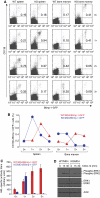
B cells from XBP-1KO/MD4 mice fail to differentiate into plasma cells. (A, B) XBP-1 deficiency leads to reduced production of plasma cells, whereas all other B-cell compartments of the XBP-1KO mice appear normal. B cells were isolated from the bone marrow, peritoneal cavity and spleen, and stained with indicated markers. Cells shown were gated on B220+ or CD19+ populations. The B-cell populations indicated in each panel are as follows: (i) pro-B cells, (ii) pre-B cells, (iii) immature B cells, (iv) pro-B cells, (v) pre-B cells, (vi) immature B cells, (vii) plasma cells, (viii) B-1 cells, (ix) transitional B cells, (x) follicular B cells, (xi) marginal zone B cells, (xii) T-1 transitional B cells, (xiii) T-2 transitional B cells, (xiv) germinal centre B cells and (xv) plasma cells. The numbers indicate the percentages of cells. (C) XBP-1WT/MD4 and XBP-1KO/MD4 mice were not immunized. XBP-1WT and XBP-1KO mice were repeatedly immunized with HEL. The levels of anti-HEL IgM in the sera were determined by ELISA.
Figure 2
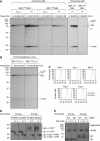
XBP-1 activation is a differentiation-dependent event in B cells, and the lack of XBP-1 leads to IRE-1 upregulation. (A) XBP-1 deficiency does not lead to accumulation of misfolded proteins. Four-day LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were treated with or without 30 μM thapsigargin for 3 h before lysis. Lysates were treated with the indicated concentrations of the cross-linker bis[sulfosuccinimidyl]suberate (BS3) and immunoblotted for calreticulin. (B) B cells purified from spleens of either XBP-1WT/MD4 and XBP-1KO/MD4 mice (upper three panels) or XBP-1WT/μS−/− and XBP-1KO/μS−/− mice (lower three panels) were cultured in LPS (20 μg/ml) to induce differentiation. Cell lysates were immunoblotted for XBP-1, p97 (AAA-ATPase) and actin. (C) XBP-1WT/μS−/− and XBP-1KO/μS−/− B cells were stimulated by LPS to induce differentiation. Lysates were immunoblotted for IRE-1, p97 and actin.
Figure 3
Transport of membrane-bound IgM and the heterodimeric Igα/Igβ to the cell surface appears to be normal in XBP-1-deficient B cells. (A) Four-day LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were labelled by 35S-[methionine] and -[cysteine] for 10 min and chased for indicated time. Cells were lysed in Triton X-114 and lysates were subjected to phase separation. The intracellular membrane-bound IgM was immunoprecipitated using the anti-μ antibody from Triton X-114-associated protein fractions, whereas the intracellular secreted IgM was immunoprecipitated from Triton X-114 supernatant fractions. The extracellular secreted IgM was immunoprecipitated from the culture media. The asterisk denotes endo-H-resistant complex glycans. (B) Four-day LPS-stimulated XBP-1WT/μS−/− and XBP-1KO/μS−/− plasmablasts were radiolabelled for 10 min and chased for indicated time. Lysates were immunoprecipitated using the anti-μ antibody. (C) Naive XBP-1WT/MD4 and XBP-1KO/MD4 B cells were stimulated by LPS for 4 days to allow differentiation. Each day cells were surface-stained by an FITC-conjugated anti-μ antibody and analysed by flow cytofluorimetry. (D) Three-day LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were lysed. Lysates were treated with either endo-H or PNGase F before immunoblotting for Igα or Igβ. CHO, CHO* and NAG represent high mannose-type glycans, complex-type glycans and _N_-acetylglucosamines, respectively. (E) Four-day LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were lysed. Lysates were cross-linked with BS3 of indicated concentrations and analysed by immunoblot for Igα or Igβ.
Figure 4
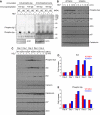
XBP-1 deficiency causes defective phosphorylation of Igα, Igβ and Syk in LPS-induced plasmablasts on antigen engagement. (A) LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were activated by trimeric HEL for 2 min and lysed immediately. Lysates were immunoprecipitated using anti-Igα and anti-Igβ antibodies. The immunoprecipitates were analysed by SDS–PAGE and immunoblotted using an anti-phospho-Igα antibody or an anti-phosphotyrosine antibody. Total lysates were immunoblotted using an anti–actin antibody as a loading control. (B) XBP-1 plays a role in the maintenance of persistent Syk phosphorylation on BCR activation. Day 4 LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were stimulated with trimeric HEL for indicated time and lysed immediately. Lysates were immunoblotted for phospho-Syk, Syk, actin, p97 and calreticulin. Quantitation of protein bands was performed, and the numbers represent relative band intensity within each gel. (C) Naive XBP-1WT/MD4 and XBP-1KO/MD4 B cells were induced to differentiate into plasmablasts by LPS. At the end of each indicated LPS stimulation time, cells were exposed to trimeric HEL for 2 min and lysed immediately. Day 0 cells were not exposed to LPS. Lysates were immunoblotted for phospho-Syk, Syk, protein disulfide isomerase (PDI), calnexin, calreticulin, p97 and actin. Note the imbalanced Syk phosphorylation between XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts from day 1 to day 4. (D) Protein bands in the Syk immunoblot in (C) were quantified and data were plotted as fold changes. (E) Protein bands in the phospho-Syk immunoblot in (C) were quantified.
Figure 5
IgM- or HEL-stimulated XBP-1-deficient B cells produce less IL-6. (A) Naive B cells were cultured in the media with or without IL-4 for 4 h, and some cells were subsequently stimulated by trimeric HEL for 2 min. Besides, naive B cells were cultured in LPS for 3 days and subsequently treated with IL-4 for another 24 h. Lysates were immunoblotted for phospho-Stat6 (upper panels) and actin (lower panels). Naive (B), 2-day LPS stimulated (C) and 4-day LPS stimulated (D) XBP-1WT/MD4 and XBP-1KO/MD4 B cells were cultured with plate-bound LPS, CpG, anti-IgM or HEL. (E) Sorted XBP-1WT/MD4 and XBP-1KO/MD4 follicular B cells (using CD1d and CD23 markers) were stimulated with LPS for 4 days and then cultured with plate-bound HEL. The level of secreted IL-6 in the culture supernatants was measured after 24 h by ELISA. *P<0.005.
Figure 6
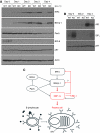
XBP-1 deficiency leads to altered expression of IRF4, Blimp-1, activation-induced cytidine deaminase (AID) and sphingosine-1-phosphate receptor (S1P1). (A) XBP-1WT/μS−/− and XBP-1KO/μS−/− B cells were cultured in LPS to induce differentiation for indicated time. Lysates were immunoblotted for IRF4, Blimp-1, Pax5, BCL6, p97 and actin. (B) XBP-1WT and XBP-1KO B cells were stimulated by LPS to induce differentiation. Lysates were immunoblotted for AID, S1P1 and p97. (C) A model that illustrates the inhibitory effect of XBP-1 on IRF4, Blimp-1 and its activating enzyme IRE-1. The red arrows indicate that the function of IRF4 and Blimp-1 to regulate plasma cell differentiation requires XBP-1.
Figure 7
XBP-1-deficient plasma cells mount only a short lived but robust antibody response due to their failure to colonize into the bone marrow. (A) XBP-1WT/MD4/Blimp-1-GFP and XBP-1KO/MD4/Blimp-1-GFP mice were immunized with HEL at the following time points: days −34, −20 and −7 (3 × immunized); days −20 and −7 (2 × immunized); day −7 (1 × immunized); or unimmunized (0 × immunized). Splenocytes and bone marrow cells from all these mice were isolated on the same day (day 0), stained for CD138 and analysed by flow cytometry. The percentage of Blimp-1-GFP-positive/CD138-positive cells is indicated in the upper right quadrants. The results are representative of two independent experiments. Note that very few cells (0.05 and 0.09%) in the bone marrow of XBP-1KO/MD4/Blimp-1-GFP mice are Blimp-1-GFP-positve and CD138-positive after reimmunization. (B) The percentages of CD138+/Blimp-1-GFP+ B cells from the spleens and bone marrow of immunized mice (shown in A) were plotted. (C) HEL-specific antibody titers in the sera from mice described in (A) were measured by ELISA. (D) Day 4 LPS-stimulated XBP-1WT/MD4 and XBP-1KO/MD4 plasmablasts were stimulated with CXCL12 for indicated time and lysed immediately. Lysates were immunoblotted for phospho-ERK1/2, ERK1/2 and actin.
Similar articles
- XBP-1-deficient plasmablasts show normal protein folding but altered glycosylation and lipid synthesis.
McGehee AM, Dougan SK, Klemm EJ, Shui G, Park B, Kim YM, Watson N, Wenk MR, Ploegh HL, Hu CC. McGehee AM, et al. J Immunol. 2009 Sep 15;183(6):3690-9. doi: 10.4049/jimmunol.0900953. Epub 2009 Aug 26. J Immunol. 2009. PMID: 19710472 Free PMC article. - The unfolded protein response of B-lymphocytes: PERK-independent development of antibody-secreting cells.
Gass JN, Jiang HY, Wek RC, Brewer JW. Gass JN, et al. Mol Immunol. 2008 Feb;45(4):1035-43. doi: 10.1016/j.molimm.2007.07.029. Epub 2007 Sep 5. Mol Immunol. 2008. PMID: 17822768 Free PMC article. - High rate of antibody secretion is not integral to plasma cell differentiation as revealed by XBP-1 deficiency.
Taubenheim N, Tarlinton DM, Crawford S, Corcoran LM, Hodgkin PD, Nutt SL. Taubenheim N, et al. J Immunol. 2012 Oct 1;189(7):3328-38. doi: 10.4049/jimmunol.1201042. Epub 2012 Aug 27. J Immunol. 2012. PMID: 22925926 - The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response.
Iwakoshi NN, Lee AH, Glimcher LH. Iwakoshi NN, et al. Immunol Rev. 2003 Aug;194:29-38. doi: 10.1034/j.1600-065x.2003.00057.x. Immunol Rev. 2003. PMID: 12846805 Review. - Regulatory mechanisms that determine the development and function of plasma cells.
Calame KL, Lin KI, Tunyaplin C. Calame KL, et al. Annu Rev Immunol. 2003;21:205-30. doi: 10.1146/annurev.immunol.21.120601.141138. Epub 2001 Dec 19. Annu Rev Immunol. 2003. PMID: 12524387 Review.
Cited by
- The unfolded protein response in immunity and inflammation.
Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. Grootjans J, et al. Nat Rev Immunol. 2016 Aug;16(8):469-84. doi: 10.1038/nri.2016.62. Epub 2016 Jun 27. Nat Rev Immunol. 2016. PMID: 27346803 Free PMC article. Review. - Anticipatory UPR Activation: A Protective Pathway and Target in Cancer.
Shapiro DJ, Livezey M, Yu L, Zheng X, Andruska N. Shapiro DJ, et al. Trends Endocrinol Metab. 2016 Oct;27(10):731-741. doi: 10.1016/j.tem.2016.06.002. Epub 2016 Jun 25. Trends Endocrinol Metab. 2016. PMID: 27354311 Free PMC article. Review. - Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains.
Volmer R, van der Ploeg K, Ron D. Volmer R, et al. Proc Natl Acad Sci U S A. 2013 Mar 19;110(12):4628-33. doi: 10.1073/pnas.1217611110. Epub 2013 Mar 4. Proc Natl Acad Sci U S A. 2013. PMID: 23487760 Free PMC article. - Germinal centres and B cell lymphomagenesis.
Basso K, Dalla-Favera R. Basso K, et al. Nat Rev Immunol. 2015 Mar;15(3):172-84. doi: 10.1038/nri3814. Nat Rev Immunol. 2015. PMID: 25712152 Review. - Interplay Between the Unfolded Protein Response and Immune Function in the Development of Neurodegenerative Diseases.
García-González P, Cabral-Miranda F, Hetz C, Osorio F. García-González P, et al. Front Immunol. 2018 Nov 2;9:2541. doi: 10.3389/fimmu.2018.02541. eCollection 2018. Front Immunol. 2018. PMID: 30450103 Free PMC article. Review.
References
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66 - PubMed
- Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J (1998) Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol 160: 4776–4787 - PubMed
- Bordier C (1981) Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem 256: 1604–1607 - PubMed
- Brunsing R, Omori-Fitzpatrick S, Weber F, Bicknell AA, Friend L, Rickert R, Niwa M (2008) B and T cell development both involve activity of the unfolded protein response pathway. J Biol Chem 283: 17954–17961 - PubMed
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases