Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach - PubMed (original) (raw)
Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach
Wei Lu et al. Neuron. 2009.
Abstract
The precise subunit composition of synaptic ionotropic receptors in the brain is poorly understood. This information is of particular importance with regard to AMPA-type glutamate receptors, the multimeric complexes assembled from GluA1-A4 subunits, as the trafficking of these receptors into and out of synapses is proposed to depend upon the subunit composition of the receptor. We report a molecular quantification of synaptic AMPA receptors (AMPARs) by employing a single-cell genetic approach coupled with electrophysiology in hippocampal CA1 pyramidal neurons. In contrast to prevailing views, we find that GluA1A2 heteromers are the dominant AMPARs at CA1 cell synapses (approximately 80%). In cells lacking GluA1, -A2, and -A3, synapses are devoid of AMPARs, yet synaptic NMDA receptors (NMDARs) and dendritic morphology remain unchanged. These data demonstrate a functional dissociation of AMPARs from trafficking of NMDARs and neuronal morphogenesis. This study provides a functional quantification of the subunit composition of AMPARs in the CNS and suggests novel roles for AMPAR subunits in receptor trafficking.
Figures
Figure 1
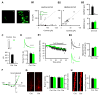
Outside-out patch (OOP) recordings of AMPAR-mediated current from CA1 pyramidal neurons in GluA2 KO and WT mice. (A) Top: Example of the strongly inwardly-rectifying I/V curve of glutamate-evoked AMPAR-mediated current from acute hippocampal slice from the germline 2–3 weeks old GluA2-KO mouse, with an RI value of 0.16. Bottom: In a subsequent glutamate application in the same OOP, held at −60 mV, ~99% of the current could be blocked by 100 nM PhTx-433. (B) Example of the linear I/V curve of glutamate-evoked current in a WT littermate, with an RI value of 0.83. Bottom: In the same OOP, glutamate-evoked current was untouched by 100 nM PhTx-433. (C) Bar graph showing average RI values for each of the genotypes: GluA2-KO mice, RI = 0.12 ± 0.02 (n = 9); WT mice, RI = 0.82 ± 0.02 (n = 15). (D) Bar graph showing the average percent block (%) of glutamate-evoked currents by 100 nM PhTx-433 in GluA2-KO mice, average % block = 97.9 ± 0.4 % (n = 6) and in WT mice, 0 % (n = 5).
Figure 2
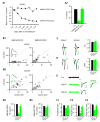
Synaptic physiology and morphology of CA1 pyramidal neurons without AMPARs. (A) Confocal images (left, low magnification; right, high magnification of the boxed area in left) show mosaic expression of Cre-GFP in the CA1 region of a typical hippocampal acute slice made from a triple GRIAXfl/fl mouse at P25 injected at P0 with rAAV-Cre-GFP. Scale bar, left, 0.2 mm; right, 20 μm. (B) Scatter plots show amplitudes of EPSCs for single pairs (open circles) and mean ± SEM (filled circles), respectively. The scatter plots represented the data recorded from acute slices (P22-P30) infected with rAAV-CRE-GFP at P0. Distributions of EPSC amplitudes show a virtual elimination of AMPAR EPSCs (B1, Control (Cnt): −127.1 ± 26.6 pA; Cre: −3.1 ± 1.0 pA; n = 13; *p < 0.001) but no change in NMDAR EPSCs (B2, control: 32.0 ± 5.1 pA; Cre: 34.7 ± 8.0 pA, n = 13; p = 0.73). (Inset in B1) Sample traces: black, control cell; green, Cre cell. (B3) Bar graph shows average AMPAR- (top) and NMDAR- (bottom) EPSCs presented in (B1-2). (C) Traces of glutamate-evoked currents from OOPs in control (black) and Cre cells (green). Bar graph shows that deletion of GluA1, -A2 and -A3 eliminated the AMPAR-mediated current (Cnt: −648.7 ± 45.2 pA; n = 23; Cre: −1.0 ± 0.7 pA; n = 8; *p < 0.001). Scale bar, 200 pA, 1 s. (D) Bar graph shows the decay time constant of NMDAR EPSCs recorded in NBQX at +40 mV (Cnt: 0.24 ± 0.01 s, n = 22; Cre: 0.23 ± 0.01 s, n = 24; p >0.05). Scale bar, 0.5 s. (E1-E2) Ifenprodil (3 μM) depressed NMDAR EPSCs recorded at +40 mV in Cnt and Cre cells to a similar extent. (E2) Traces of NMDAR EPSCs from the two groups of cells before and 30 min after ifenprodil application were shown on the right. Bar graph shows the average percentage of NMDAR EPSCs remaining after ifenprodil application. (Cnt: 66.8% ± 3.7%, n = 4; Cre: 74% ± 4.8%, n = 5; p >0.05). Scale bar, 50 pA, 0.1 s. (F) I/Vs of synaptic NMDARs. NMDAR EPSCs were recorded at various holding potentials (−80, −60, −40, −20, 0, +20, +40mV) with 4 mM Mg2+. Junction potentials have been corrected. (G) Representative confocal stacks from Cnt and Cre cells. Bar graph in right shows average number dendritic branch points and dendritic length (Cnt: n = 10; Cre: n = 8; p >0.05). Scale bar, 20 μm. (H) Representative confocal stacks of 20 μm secondary apical dendrites from Cnt and Cre cells. Bar graph in right shows average spine density (Cnt: n = 11; Cre: n = 11; p > 0.05). Scale bar, 2 μm. (A-H) The recordings and anatomy were made from acute slices (P20-P30) from animals injected at P0 with rAAV-Cre-GFP.
Figure 3
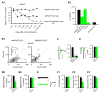
Excitatory synaptic transmission at CA1 pyramidal neurons is mediated primarily by GluA1A2 heteromers. (A1-2) The time course for changes in AMPAR EPSCs in hippocampal slice cultures from GRIA1fl/fl mice after transfection of Cre-IRES-GFP. For ΔGluA1: ratio of AMPAR-EPSCs (closed circles: 3-5d, 1.02; 6d, 0.75; 7-8d, 0.43; 9-10d, 0.37; 11-12d, 0.28; 12-14d, 0.23; >14d, 0.21), and ratio of NMDAR-EPSCs (closed diamonds: 3-5d, 0.98; 6d, 1.15; 7-8d, 1.11; 9-10d, 1.11; 11-12d, 1.16; 12-14d, 0.92; >14d, 1.09) from transfected cells to neighboring control cells, respectively. (A2) Bar graph shows the percentage of AMPAR EPSCs (21.2 ± 3.1%; n = 15; *p < 0.0001) and NMDAR EPSCs (104.8 ± 17.6%; n = 14; p = 0.53) to controls. (B1-4) Scatter plots (B1-2) and bar graphs (B3-4) show amplitudes of EPSCs for single pairs (open circles) and mean ± SEM (filled circles) for _GRIA1fl/fl_ (B1, pooled data from acute slices (P19-P24) from animals injected at P0-P2 and from hippocampal slice cultures.) and _GRIA3fl/fl_ (B2, data from acute slices (P20-P25) from animals injected at P0-P2), respectively. (Inset in B1-2) Sample traces: black, control; green, Cre. (B3) EPSC amplitudes show a significant reduction in AMPAR EPSCs for the deletion of either subunit (ΔGluA1, Cnt, −77.7 ± 12.7 pA; Cre, −15.1 ± 2.4 pA; n = 31, *p < 0.0001; ΔGluA3, Cnt: −56.4 ± 6.0 pA; Cre: −47.2 ± 5.6 pA; n = 19; *p < 0.05). (B4) There was no change in the NMDAR EPSCs (GluA1, Cnt: 40.0 ± 9.4 pA; Cre: 33.6 ± 6.9 pA, n = 29; p = 0.31; ΔGluA3, Cnt: 40.4 ± 7.7 pA; Cre: 39.0 ± 7.8 pA, n = 19; p = 0.97). (C–D) Bar graphs show average RI (C) (Cnt: 0.99 ± 0.03, n = 30; ΔGluA1: 1.02 ± 0.08, n = 14; p = 0.63; ΔGluA3: 1.06 ± 0.04, n = 15; p = 0.15), and average paired-pulse ratio (PPR, D) (Cnt: n = 84; ΔGluA1: n = 40; ΔGluA3: n = 9; p > 0.05 for both conditions). Left were sample traces. (E) Sample traces of mEPSCs shown at a low gain and sweep speed (traces on left) and averaged mEPSCs at a high gain and sweep speed (traces on right). Control trace (black) has been superimposed on the trace from a Cre cell. Scale bar, 5 pA, 10 ms. mEPSCs were recorded from acute hippocampal slices (P20-P27) from animals injected at P0-P2. (F1) Bar graphs show mEPSCs amplitude (Cnt: −10.5 ± 0.4 pA; ΔGluA1: −7.9 ± 0.5 pA; *p < 0.001; ΔGluA3: −10.7 ± 0.1 pA; p = 0.77), (F2) frequency (Cnt: 0.08 ± 0.03 Hz; ΔGluA1: 0.08 ± 0.01; p* < 0.001; ΔGluA3: 0.27 ± 0.05 Hz, p = 0.68) and (F3) decay (Cnt: 11.30 ± 0.49 ms; ΔGluA1: 7.73 ± 1.41 ms; *p < 0.01; ΔGluA3: 11.60 ± 1.20 ms; p = 0.81). n = 22, 10 and 20 for Cnt, ΔGluA1 and ΔGluA3, respectively.
Figure 4
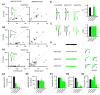
AMPARs adjust rapidly to the deletion of GluA2. (A1-2) The time course for the changes in synaptic transmission in hippocampal slice cultures from GRIA2fl/fl mice after transfection of Cre-IRES-GFP. Ratio of RI (open circles: 3-5d, 0.95; 6d, 0.99; 7-8d, 0.71; 9-10d, 0.60; 11-12d, 0.34; 12-14d, 0.16; >14d, 0.15), ratio of AMPAR EPSCs (closed circle: 3-5d, 0.96; 6d, 0.49; 7-8d, 0.57; 9-10d, 0.56; 11-12d, 0.50; 12-14d, 0.57; >14d, 0.51), and ratio of NMDAR EPSCs (closed diamonds: 3-5d, 1.08; 6d, 1.01; 7-8d, 1.06; 9-10d, 1.04; 11-12d, 0.99; 12-14d, 1.01; >14d, 1.10) from transfected cells to neighboring control cells, respectively. Open square shows RI from CA1 pyramidal neurons from germ-line GluA2 KO mice (0.13 ± 0.02, n = 5). (A2) Graph shows the percentage of the average AMPAR EPSCs (51.7 ± 5.2%; n = 86; *p < 0.0001), NMDAR EPSCs (97.8 ± 13.2%; n = 64; p = 0.81), RI (15.0 ± 1.8%; n = 19; *p < 0.0001) from transfected cells or GluA2 KO cells (13.3 ± 2.0%; n = 5; *p < 0.01) to control cells. (B1-3) Scatter plots (B1) and bar graphs (B2-3) show amplitudes of EPSCs for single pairs (open circles) and mean ± SEM (filled circles) for _GRIA2fl/fl_. (Inset in B1) Sample traces: black, control; green, Cre. (B2) EPSC amplitudes show a significant reduction in the AMPAR EPSCs (Cnt: −66.2 ± 3.8 pA; Cre: −34.2 ± 2.5 pA; n = 86; *p < 0.0001. (B3) There was no change in the NMDAR EPSCs (GluA2, Cnt: 40.0 ± 3.7 pA; Cre: 39.1 ± 3.4 pA, n = 64; p = 0.81. The data were pooled from acute hippocampal slices (P13-P17) from animals injected at P0-P2 and from hippocampal slice cultures. (C-D) Bar graphs show average RI (C) (Cnt: 0.99 ± 0.03, n = 30; ΔGluA2: 0.15 ± 0.02, n = 19; *p < 0.001) and average PPR (D) (Cnt: n = 84; ΔGluA2: n = 29; p > 0.05). Left were sample traces. (E) Sample traces of mEPSCs shown at a low gain and sweep speed (traces on left) and averaged mEPSCs at a high gain and sweep speed (traces on right). Control trace (black) has been superimposed on the trace from a Cre cell. Scale bar, 5 pA, 10 ms. mEPSCs were recorded from acute hippocampal slices (P13-P18) from animals injected at P0-P2. (F1) Bar graphs show mEPSCs amplitude (Cnt: −10.51 ± 0.37 pA; ΔGluA2: 11.08 ± 0.65 pA; p = 0.42), (F2) frequency (Cnt: 0.28 ± 0.03 Hz; ΔGluA2: 0.16 ± 0.03 Hz; *p < 0.001) and (F3) decay (Cnt: 11.30 ± 0.49 ms; ΔGluA2: 9.75 ± 1.14 ms; p = 0.27). n = 22 and 17 for Cnt and ΔGluA2, respectively.
Figure 5
Deletion of GluA2A3, GluA1A3 or GluA1A2 in CA1 pyramidal cells. (A1–A5) Scatter plots (A1-3) and bar graphs (A4-5) show amplitudes of EPSCs for single pairs (open circles) and mean ± SEM (filled circles) for GRIA2A3fl/fl (A1), GRIA1A3fl/fl (A2), GRIA1A2fl/fl (A3), respectively. (A4) The amplitudes AMPAR EPSCs were significantly reduced in all three cases (ΔGluA2A3, Cnt: −58.1 ± 11.4 pA; Cre: −24.9 ± 3.3 pA; n = 14; *p < 0.01; ΔGluA1A3, Cnt: −128.4 ± 19.7 pA; Cre: −15.6 ± 3.10 pA; n = 12; *p < 0.001; ΔGluA1A2, Cnt: −84.3 ± 10.1 pA; Cre: −4.9 ± 0.8 pA; n = 24; *p < 0.001). (A5) No change in the size of NMDAR EPSCs was observed (ΔGluA2A3, Cnt: 40.3 ± 7.4 pA; Cre: 38.0 ± 6.3 pA, n = 12; p = 0.82; ΔGluA1A3, Cnt: 49.2 ± 11.7 pA; Cre: 49.0 ± 13.7 pA, n = 11; p = 0.99; ΔGluA1A2; Cnt: 36.3 ± 5.8 pA; Cre: 31.0 ± 4.2 pA, n = 23; p = 0.31). (Inset in A1-3) Sample traces: black, control; green, Cre. (B-C) Bar graphs show average RI (B) (Cnt: 0.99 ± 0.03, n = 30; ΔGluA2A3: 0.14 ± 0.02, n = 13; *p < 0.001; ΔGluA1A3: 1.06 ± 0.2, n = 5; p = 0.59; ΔGluA1A2: 0.1 ± 0.02, n = 6; *p < 0.001), and average PPR (C) (Cnt: n = 84; ΔGluA2A3: n = 14; ΔGluA1A3: n = 6; ΔGluA1A2: n = 11; p > 0.05 for each conditions). Left were sample traces. For GRIA1A2fl/fl cells, the stimulus was increased to record measureable EPSCs and only recordings from the Cre cell were shown. (D) Sample recordings of mEPSCs at low gain and sweep speed (traces on left) and averaged mEPSCs at high gain and sweep speed (traces on right) Control trace (black) has been superimposed on the trace from a Cre cell. Scale bar, 5 pA, 10 ms. (E1) Bar graphs show mEPSC amplitude (top; Cnt: −10.51 ± 0.37 pA; ΔGluA2A3: −10.56 ± 0.60 pA; p = 0.93; ΔGluA1A3: −7.21 ± 0.36 pA; *p < 0.001; ΔGluA1A2: −6.79 ± 0.20 pA; *p < 0.001), (E2) frequency (middle; Cnt: 0.28 ± 0.03 Hz; ΔGluA2A3: 0.17 ± 0.05 Hz; *p < 0.005; ΔGluA1A3: 0.03 ± 0.01 Hz; *p < 0.001; ΔGluA1A2: 0.06 ± 0.01 Hz, *p < 0.001) and (E3) decay (bottom, Cnt: 11.30 ± 0.49 ms; ΔGluA2A3: 10.18 ± 1.2 ms; p = 0.33; ΔGluA1A3: 14.70 ± 0.71 ms; *p < 0.01; ΔGluA1A2: 4.20 ± 0.71 ms; *p < 0.001). n = 22, 14, 7 and 9 for Cnt, ΔGluA2A3, ΔGluA1A3 and ΔGluA1A2, respectively. (A-E) the recordings were made from acute hippocampal slices (P20-P27) from animals injected at P0-P1.
Figure 6
Analysis of extrasynaptic AMPARs. (A) Sample traces of AMPAR currents from OOPs from uninfected control (black) and Cre (green) cells from CA1 pyramidal neurons from various genetic backgrounds. Scale 200 pA, 1s. The recordings were made from acute hippocampal slices (P13-P17 for ΔGluA2 and P20-P28 for all other genetic backgrounds) from animals injected at P0-P2. (B) I/V curves of AMPAR currents from OOPs. Control: black; Cre: green. Deletion of the GluA2 subunit, but not other subunits, caused strong inward rectification of the evoked current. Bar graph at the bottom showing the RI for each condition (Cnt: 0.85 ± 0.02, n = 8; ΔGluA1: 0.81 ± 0.04, n = 5; p = 0.39; ΔGluA2: 0.09 ± 0.01, n = 6; *p < 0.001; ΔGluA3: 0.80 ± 0.03, n = 5; p = 0.22; ΔGluA2A3: 0.10 ± 0.02, n = 6; *p < 0.001; ΔGluA1A2: 0.15 ± 0.03, n = 5; *p < 0.001). (C) Summary bar graph shows consequences of deletion of respective gene (s) on AMPAR current from OOPs (Cnt: −648.7 ± 45.2 pA, n = 23; ΔGluA1: −35.3 ± 13.1 pA, n = 16, *p < 0.001; ΔGluA2: −684.3 ± 92.2 pA, n = 11, p = 0.70; ΔGluA3: −674.2 ± 63.5 pA, n = 13, p = 0.74; ΔGluA2A3: −656.8 ± 76.3 pA, n = 14, p = 0.92; ΔGluA1A3: −2.5 ± 1.0 pA, n = 14, *p < 0.001; ΔGluA1A2: −24.1 ± 5.2 pA, n = 25, *p < 0.001; ΔGluA1A2A3: −1.01 ± 0.65 pA, n = 8, *p < 0.001). (D) Summary bar graph shows consequences of deletion of respective gene (s) on AMPAR EPSCs (% control: ΔGluA1: 19.4 ± 3.1%, n = 31, *p < 0.001; ΔGluA2: 51.7 ± 3.8%, n = 86, *p < 0.001; ΔGluA3: 83.8 ± 1.0%, n = 19, *p < 0.05; ΔGluA2A3: 42.8 ± 5.2%, n = 14, *p < 0.001; ΔGluA1A3: 12.1 ± 2.4%, n = 12, *p < 0.001; ΔGluA1A2: 5.7 ± 1.4%, n = 24, *p < 0.001; ΔGluA1A2A3: 2.4 ± 0.6%, n = 13, *p < 0.001). (E) Models for AMPAR compositions at synaptic and extrasynaptic membranes. At CA1 pyramidal neurons, ~80% synaptic AMPARs are GluA1A2 heteromers, and ~16% synaptic AMPARs are GluA2A3 heteromers. On the other hand, ~95% extrasynaptic AMPARs are GluA1A2 heteromers.
Comment in
- AMPA receptor subunits get their share of the pie.
Béïque JC, Huganir RL. Béïque JC, et al. Neuron. 2009 Apr 30;62(2):165-8. doi: 10.1016/j.neuron.2009.04.016. Neuron. 2009. PMID: 19409261
Similar articles
- Activity patterns govern synapse-specific AMPA receptor trafficking between deliverable and synaptic pools.
Kielland A, Bochorishvili G, Corson J, Zhang L, Rosin DL, Heggelund P, Zhu JJ. Kielland A, et al. Neuron. 2009 Apr 16;62(1):84-101. doi: 10.1016/j.neuron.2009.03.001. Neuron. 2009. PMID: 19376069 Free PMC article. - AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors.
Sanderson JL, Gorski JA, Gibson ES, Lam P, Freund RK, Chick WS, Dell'Acqua ML. Sanderson JL, et al. J Neurosci. 2012 Oct 24;32(43):15036-52. doi: 10.1523/JNEUROSCI.3326-12.2012. J Neurosci. 2012. PMID: 23100425 Free PMC article. - Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses.
Kakegawa W, Tsuzuki K, Yoshida Y, Kameyama K, Ozawa S. Kakegawa W, et al. Eur J Neurosci. 2004 Jul;20(1):101-10. doi: 10.1111/j.1460-9568.2004.03461.x. Eur J Neurosci. 2004. PMID: 15245483 - Synaptic AMPA receptor composition in development, plasticity and disease.
Henley JM, Wilkinson KA. Henley JM, et al. Nat Rev Neurosci. 2016 Jun;17(6):337-50. doi: 10.1038/nrn.2016.37. Epub 2016 Apr 15. Nat Rev Neurosci. 2016. PMID: 27080385 Review. - The AMPAR subunit GluR2: still front and center-stage.
Tanaka H, Grooms SY, Bennett MV, Zukin RS. Tanaka H, et al. Brain Res. 2000 Dec 15;886(1-2):190-207. doi: 10.1016/s0006-8993(00)02951-6. Brain Res. 2000. PMID: 11119696 Review.
Cited by
- Regulation of the Postsynaptic Compartment of Excitatory Synapses by the Actin Cytoskeleton in Health and Its Disruption in Disease.
Stefen H, Chaichim C, Power J, Fath T. Stefen H, et al. Neural Plast. 2016;2016:2371970. doi: 10.1155/2016/2371970. Epub 2016 Apr 5. Neural Plast. 2016. PMID: 27127658 Free PMC article. Review. - Distinct Cell Surface Expression Patterns of N-Glycosylation Site Mutants of AMPA-Type Glutamate Receptor under the Homo-Oligomeric Expression Conditions.
Morise J, Yamamoto S, Midorikawa R, Takamiya K, Nonaka M, Takematsu H, Oka S. Morise J, et al. Int J Mol Sci. 2020 Jul 19;21(14):5101. doi: 10.3390/ijms21145101. Int J Mol Sci. 2020. PMID: 32707739 Free PMC article. - FoxO6 regulates memory consolidation and synaptic function.
Salih DA, Rashid AJ, Colas D, de la Torre-Ubieta L, Zhu RP, Morgan AA, Santo EE, Ucar D, Devarajan K, Cole CJ, Madison DV, Shamloo M, Butte AJ, Bonni A, Josselyn SA, Brunet A. Salih DA, et al. Genes Dev. 2012 Dec 15;26(24):2780-801. doi: 10.1101/gad.208926.112. Epub 2012 Dec 7. Genes Dev. 2012. PMID: 23222102 Free PMC article. - O brother, wherefore are thou? Calcium-permeable AMPA receptors make an appearance in adult status epilepticus.
Benke T. Benke T. Epilepsy Curr. 2013 Jan;13(1):32-4. doi: 10.5698/1535-7511-13.1.32. Epilepsy Curr. 2013. PMID: 23447737 Free PMC article. No abstract available. - RNF220 is an E3 ubiquitin ligase for AMPA receptors to regulate synaptic transmission.
Ma P, Wan LP, Li Y, He CH, Song NN, Zhao S, Wang H, Ding YQ, Mao B, Sheng N. Ma P, et al. Sci Adv. 2022 Sep 30;8(39):eabq4736. doi: 10.1126/sciadv.abq4736. Epub 2022 Sep 30. Sci Adv. 2022. PMID: 36179027 Free PMC article.
References
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. - PubMed
- Ayalon G, Segev E, Elgavish S, Stern-Bach Y. Two regions in the N-terminal domain of ionotropic glutamate receptor 3 form the subunit oligomerization interfaces that control subtype-specific receptor assembly. J Biol Chem. 2005;280:15053–15060. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous