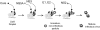Trans-complementation of an NS2 defect in a late step in hepatitis C virus (HCV) particle assembly and maturation - PubMed (original) (raw)
Trans-complementation of an NS2 defect in a late step in hepatitis C virus (HCV) particle assembly and maturation
MinKyung Yi et al. PLoS Pathog. 2009 May.
Abstract
Recent studies using cell culture infection systems that recapitulate the entire life cycle of hepatitis C virus (HCV) indicate that several nonstructural viral proteins, including NS2, NS3, and NS5A, are involved in the process of viral assembly and release. Other recent work suggests that Ser-168 of NS2 is a target of CK2 kinase-mediated phosphorylation, and that this controls the stability of the genotype 1a NS2 protein. Here, we show that Ser-168 is a critical determinant in the production of infectious virus particles. Substitution of Ser-168 with Ala (or Gly) ablated production of infectious virus by cells transfected with a chimeric viral RNA (HJ3-5) containing core-NS2 sequences from the genotype 1a H77 virus within the background of genotype 2a JFH1 virus. An S168A substitution also impaired production of virus by cells transfected with JFH1 RNA. This mutation did not alter polyprotein processing or genome replication. This defect in virus production could be rescued by expression of wt NS2 in trans from an alphavirus replicon. The trans-complementing activities of NS2 from genotypes 1a and 2a demonstrated strong preferences for rescue of the homologous genotype. Importantly, the S168A mutation did not alter the association of core or NS5A proteins with host cell lipid droplets, nor prevent the assembly of core into particles with sedimentation and buoyant density properties similar to infectious virus, indicating that NS2 acts subsequent to the involvement of core, NS5A, and NS3 in particle assembly. Second-site mutations in NS2 as well as in NS5A can rescue the defect in virus production imposed by the S168G mutation. In aggregate, these results indicate that NS2 functions in trans, in a late-post assembly maturation step, perhaps in concert with NS5A, to confer infectivity to the HCV particle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
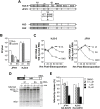
Figure 1. A single mutation in NS2 disrupts infectious virus production without affecting viral RNA replication.
(A) Structure of the chimeric HJ3-5 and JFH1 HCV RNAs. HJ3-5 has two compensatory mutations, located within the E1 (YH) and NS3 (QL) sequences from its HJ3 parent . Also shown is the HJ2 chimera, in which the NS2 protein is itself chimeric. The H77 sequence is represented by the shaded boxes, while the JFH1 sequence is open. The location of the putative CK2 phosphorylation site and the S168A mutation within NS2 is highlighted. (B) Infectious virus titers present in supernatant fluids and cell lysates 2 days following transfection of FT3-7 cells with the JFH1 and HJ3-5 RNAs, with (“SA”) or without (“wt”) the S168A mutation. The limit of detection of infectious virus, determined in a replication focus-forming assay (10 FFU/ml), is indicated by the dashed line. (C) Replication of HJ3-5 (left panel) and JFH1 (right panel) RNAs with or without the SA mutation. “GND” represents a mutant genome defective in RNA replication due to the mutation of GDD to GND within NS5B polymerase active site. Viral RNA abundance was measured by quantitative RT-PCR assay of 100 ng of total RNA derived from cell lysates harvested at 4, 24, 48, 72, and 96 h following transfection of the indicated RNA into FT3-7 cells. The data represent a triplicate analysis, ±S.D. (D) Auto-processing of the NS2-NS3 junction site in NS2-NS3-NS4A polyprotein segments translated in vitro in reticulocyte lysates programmed with wt versus the S168A mutant HJ3-5 RNA. The organization of the proteins translated in vitro are shown at the top. Below are shown the protein products of in vitro translation reactions (15–120 min reaction time), separated by SDS-PAGE. There was no difference in the rate of generation of the mature NS2 protein over time in the presence or absence of the SA mutation. (E) Treatment with sub-toxic concentrations of the CK2 inhibitor DMAT does not reduce virus yields from JFH1 or HJ3-5-transfected cells. In addition to the presence or absence of the NS2 SA mutation, the RNAs transfected in this experiment each contained an S457D mutation in NS5A, which eliminates the need for CK2 phosphorylation of this viral protein. The inhibitor was added to the media from 4 to 24 h post-transfection; virus yields were determined by titration of supernatant fluids collected at 48 h.
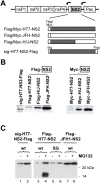
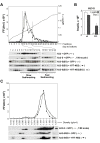
Figure 2. Stability of NS2 proteins expressed by subgenomic VEE replicons.
(A) At the top is shown the organization of subgenomic VEE replicons expressing the H77, JFH1, and chimeric HJ2 (“H/J”) NS2 protein. Arrows indicate the VEE subgenomic promoter, while “Pac” indicates the puromycin resistance gene. Shown below are the different NS2 proteins expressed by this series of replicons; each contains either an N- or C-terminal Flag (or Myc) tag. (B) Immunoblot detection of NS2 protein using anti-Flag (left panel) or anti-Myc (right panel) antibodies. With the exception of sig-H77-NS2-Flag, which has a Flag tag at the C-terminal end of the NS2 protein, all of the other NS2 proteins have a Flag or Myc tag at the N-terminus. (C) Treatment with the proteasome inhibitor MG132 enhances the abundance of H77 NS2 (both wt and to a lesser extent the S168G mutant, “SG”) but not JFH1 NS2 expressed by VEE replicon cell lines.
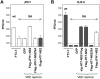
Figure 3. Genotype-specific _trans-_complementation of the NS2 S168A-mediated defect in infectious virus production.
(A) Extracellular virus yields (FFU/ml) determined three days after transfection of JFH1 RNA, with or without the S168A mutation (“SA”), into normal FT3-7 cells or VEE replicon cells expressing GFP, Flag-JFH1-NS2, Flag-JFH1-NS2/SA, or Flag-H77-NS2. (B) Extracellular virus yields (FFU/ml) determined in a similar fashion following transfection of the HJ3-5 RNA with or without the S168A mutation into normal FT3-7 cells, or VEE replicon cells expressing GFP, the homologous NS2 sequence with or without the SA mutation, or the heterologous wild-type NS2 sequence (sig-H77-NS2-Flag, Flag-H77-NS2, Flag-H77-NS2/SA, or Flag-JFH1-NS2 replicon cells).
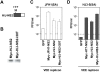
Figure 4. The I30T mutation in NS2 enhances the efficiency of trans complementation in a genotype-specific manner.
(A) Organization of the chimeric H/J-NS2 replicon expression product, showing the location of the compensatory I30T mutation in the H77-derived sequence ,. See also Figure 2A. (B) Immunoblot detection of NS2 protein using anti-Myc antibodies. (C,D) Infectious virus yields after transfection of (C) JFH1(SA) or (D) HJ3-5(SA) RNA into VEE replicon cells expressing GFP, or Myc-H/J-NS2 with or without the I30T mutation. Extracellular virus yields were determined three days following transfection. The detection limit of the infectivity assay is indicated by the broken line.
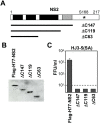
Figure 5. C-terminal deletion mutants of NS2 are incapable of complementing the S168A defect in virus production.
(A) NS2 C-terminal deletion mutants expressed by modified VEE-Flag-H77-NS2 replicons. The black and grey shaded areas indicate four potential transmembrane domains within NS2 that have been reported previously . The grey sequence area needs to be located within the cytoplasm to function as part of the NS2-NS3 protease . The position of Ser-168 is indicated. (B) Immunoblots of full-length and C terminally truncated versions of the H77 NS2 protein expressed by the VEE replicon cell lines. (C) Extracellular infectious virus yields determined three days after transfection of the mutant HJ3-5(SA) RNA into the VEE replicon cell lines expressing wt and C-terminal deletion mutants of H77 NS2.
Figure 6. Detection of fast-sedimenting, core-containing HJ3-5(SG) particles with physical properties similar to infectious virus.
(A) Rate zonal centrifugation of cell lysates derived from VEE replicon cell lines expressing GFP, Flag-H77-NS2, or Flag-H77-NS2(SG) following transfection with the HJ3, HJ3-5, and HJ3-5(SG) RNA. A total of 40 fractions were collected from each gradient: the titer of infectious virus in fractions from HJ3-5 transfected cells was reduced in scale 40-fold to allow it to be plotted on the same graph as the _trans_-complemented infectious particles present in lysates of Flag-H77-NS2 cells transfected with the mutant HJ3-5(SG) RNA. Cell lysates were prepared three days following transfection by multiple rounds of freeze-thawing. The top panel shows the sedimentation profile of infectious HJ3-5 and trans-complemented HJ3-5(SG)-derived particles. The bottom panel shows immunoblot detection of core protein in fractions 1–20 of the gradients. The fractions containing the peak infectivity are indicated with dashed lines. Slow-sedimenting core antigen was present in fractions 6–13, while fast-sedimenting particles were found in fractions 14–19. (B) Core antigen measured by a quantitative ELISA assay in cell lysates or supernatant fluid from FT3-7 cells transfected with HJ3-5 RNA, with or without the S168G mutation. (C) Equilibrium density gradient ultracentrifugation of particles present in VEE replicon cell lysates expressing GFP or Flag-H77-NS2, three days post-transfection of the HJ3-5 or HJ3-5(SG) RNA. Infectious virus titer of each fraction is shown in the top panel, with the scale reduced 80-fold for HJ3-5 to allow it to be plotted in the same graph as the trans-complemented HJ3-5(SG) –derived virus. The relative distribution of core antigen present in each gradient fraction, as determined by immunoblotting, is shown in the lower panel.
Figure 7. Mutation of NS2 S168 does not perturb the co-localization of core and NS5A on the lipid droplet.
(A) FT3-7 cells were transfected with HJ3-5 or HJ3-5(SG) RNA, and two days later washed and fixed, as described in Materials and Methods, then labelled with antibodies specific for core protein (green) and NS5A (red) followed by examination with a Zeiss LSM 510 Meta microscope. Neutral lipid staining is shown in blue. At the right is an enlarged area from the merged image showing enlarged views of solitary lipid droplets (see box). (B) HJ3-5(SA) RNA was transfected into the VEE replicon cell line expressing FlagJFH1-NS2. The intracellular localization of core and NS5A was determined as described in (A).
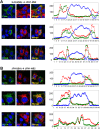
Figure 8. Confocal microscopic imaging of intracellular NS2 distribution.
NS2 protein co-localizes with E2 but not core protein. VEE replicon cells expressing FlagJFH1-NS2 were transfected with (A) HJ3-5(SA) or (B) JFH1(SA) RNA and fixed and labeled two days later with antibodies specific for core protein (green, upper panel), E2 (green, middle panel), NS5A (green, lower panel), or NS2 (red). Slides were subsequently examined with a Zeiss LSM 510 Meta microscope. At the right are line scan results obtained using MetaMorph software depicting a linear trace of the fluorescence intensity of individual pixels along a segment of the white arrow overlaying the merged image. The Y axis represents average pixel intensity while the X axis represents distance from the origin. Red, green, and blue traces correspond to the color of the fluorophores shown in merged images. This analysis revealed a high level of colocalization between NS2 and E2, but only partial colocalization of NS2 and NS5A.
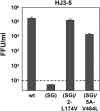
Figure 9. Second-site mutations in NS2 or NS5A rescue infectious virus production by HJ3-5(SG) RNA.
Extracellular virus yields were determined two days post-transfection of FT3-7 cells with the indicated RNAs. Results shown represent the average from two independent experiments.
Figure 10. A model for sequential involvement of non-structural proteins in HCV assembly, maturation, and egress.
Particle assembly follows the recruitment of core, followed by NS5A and NS3 to the surface of lipid droplets. Such particles are noninfectious, however, and must undergo a maturation step mediated by NS2 (most likely in concert with NS5A) prior to becoming infectious and exiting the cell. See the Discussion for details.
Similar articles
- Analysis of hepatitis C virus core/NS5A protein co-localization using novel cell culture systems expressing core-NS2 and NS5A of genotypes 1-7.
Galli A, Scheel TKH, Prentoe JC, Mikkelsen LS, Gottwein JM, Bukh J. Galli A, et al. J Gen Virol. 2013 Oct;94(Pt 10):2221-2235. doi: 10.1099/vir.0.053868-0. Epub 2013 Aug 1. J Gen Virol. 2013. PMID: 23907394 - Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly.
Appel N, Zayas M, Miller S, Krijnse-Locker J, Schaller T, Friebe P, Kallis S, Engel U, Bartenschlager R. Appel N, et al. PLoS Pathog. 2008 Mar 28;4(3):e1000035. doi: 10.1371/journal.ppat.1000035. PLoS Pathog. 2008. PMID: 18369481 Free PMC article. - Adaptive Mutations Enhance Assembly and Cell-to-Cell Transmission of a High-Titer Hepatitis C Virus Genotype 5a Core-NS2 JFH1-Based Recombinant.
Mathiesen CK, Prentoe J, Meredith LW, Jensen TB, Krarup H, McKeating JA, Gottwein JM, Bukh J. Mathiesen CK, et al. J Virol. 2015 Aug;89(15):7758-75. doi: 10.1128/JVI.00039-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995244 Free PMC article. - Processing and functions of Hepatitis C virus proteins.
Suzuki R, Suzuki T, Ishii K, Matsuura Y, Miyamura T. Suzuki R, et al. Intervirology. 1999;42(2-3):145-52. doi: 10.1159/000024973. Intervirology. 1999. PMID: 10516468 Review. - Hepatitis C virus: viral proteins on the move.
McLauchlan J. McLauchlan J. Biochem Soc Trans. 2009 Oct;37(Pt 5):986-90. doi: 10.1042/BST0370986. Biochem Soc Trans. 2009. PMID: 19754437 Review.
Cited by
- Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture.
Gastaminza P, Dryden KA, Boyd B, Wood MR, Law M, Yeager M, Chisari FV. Gastaminza P, et al. J Virol. 2010 Nov;84(21):10999-1009. doi: 10.1128/JVI.00526-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686033 Free PMC article. - Last stop before exit - hepatitis C assembly and release as antiviral drug targets.
Tews BA, Popescu CI, Dubuisson J. Tews BA, et al. Viruses. 2010 Aug;2(8):1782-1803. doi: 10.3390/v2081782. Epub 2010 Aug 24. Viruses. 2010. PMID: 21994707 Free PMC article. - Y-box-binding protein 1 interacts with hepatitis C virus NS3/4A and influences the equilibrium between viral RNA replication and infectious particle production.
Chatel-Chaix L, Melançon P, Racine MÈ, Baril M, Lamarre D. Chatel-Chaix L, et al. J Virol. 2011 Nov;85(21):11022-37. doi: 10.1128/JVI.00719-11. Epub 2011 Aug 17. J Virol. 2011. PMID: 21849455 Free PMC article. - Hepatitis C virus: Morphogenesis, infection and therapy.
Morozov VA, Lagaye S. Morozov VA, et al. World J Hepatol. 2018 Feb 27;10(2):186-212. doi: 10.4254/wjh.v10.i2.186. World J Hepatol. 2018. PMID: 29527256 Free PMC article. Review. - Production of hepatitis C virus lacking the envelope-encoding genes for single-cycle infection by providing homologous envelope proteins or vesicular stomatitis virus glycoproteins in trans.
Li R, Qin Y, He Y, Tao W, Zhang N, Tsai C, Zhou P, Zhong J. Li R, et al. J Virol. 2011 Mar;85(5):2138-47. doi: 10.1128/JVI.02313-10. Epub 2010 Dec 15. J Virol. 2011. PMID: 21159872 Free PMC article.
References
- Alter HJ. HCV natural history: The retrospective and prospective in perspective. J Hepatol. 2005;43:550–552. - PubMed
- Pawlotsky JM. Pathophysiology of hepatitis C virus infection and related liver disease. Trends Microbiol. 2004;12:96–102. - PubMed
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. - PubMed
- Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI040035/AI/NIAID NIH HHS/United States
- R01-AI075090/AI/NIAID NIH HHS/United States
- N01 AI025488/AI/NIAID NIH HHS/United States
- U19-AI40035/AI/NIAID NIH HHS/United States
- R01 AI075090/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous