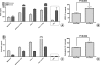Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma - PubMed (original) (raw)
Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma
Mert Erkan et al. Neoplasia. 2009 May.
Abstract
Background and aims: Although both cancer and stellate cells (PSCs) secrete proangiogenic factors, pancreatic cancer is a scirrhous and hypoxic tumor. The impact of cancer-PSCs interactions on angiogenesis was analyzed.
Methods: Expression of periostin, CD31, and alpha-smooth muscle actin was assessed by immunohistochemistry. Human PSCs and cancer cells were cultivated under normoxia and hypoxia alone, or in coculture, to analyze the changes in their angiogenic and fibrogenic attributes, using enzyme-linked immunosorbent assay, immunoblot, and quantitative polymerase chain reaction analyses and growth of cultured endothelial cells in vitro.
Results: On the invasive front of the activated stroma, PSCs deposited a periostin-rich matrix around the capillaries in the periacinar spaces. Compared with the normal pancreas, there was a significant reduction in the microvessel density in chronic pancreatitis (five-fold, P < .001) and pancreatic cancer (four-fold, P < .01) tissues. In vitro, hypoxia increased PSCs' activity and doubled the secretion of periostin, type I collagen, fibronectin, and vascular endothelial growth factor (VEGF). Cancer cells induced VEGF secretion of PSCs (390 +/- 60%, P < .001), whereas PSCs increased the endostatin production of cancer cells (210 +/- 14%, P < .001) by matrix metalloproteinase-dependent cleavage. In vitro, PSCs increased the endothelial cell growth, whereas cancer cells alone, or their coculture with PSCs, suppressed it.
Conclusions: Although PSCs are the dominant producers of VEGF and increase endothelial cell growth in vitro, in the peritumoral stroma, they contribute to the fibrotic/hypoxic milieu through abnormal extracellular matrix deposition and by amplifying endostatin production of cancer cells.
Figures
Figure 1
Localization of CD31, α-SMA, and periostin in normal and diseased pancreatic tissues: Immunohistochemical analysis was carried out using consecutive tissue sections of the normal pancreas. Sections were probed with antibodies against CD31 (A; original magnification, x200) for endothelial cells and against α-SMA (B) for smooth muscle cells and PSCs. Analysis of periostin expression in CP tissues (C–E; original magnification, x100): The PSCs in the periacinar spaces are marked by their periostin expression (C, arrows). Note that periostin-positive PSCs do not yet express α-SMA (D), and periostin has not yet been deposited in the periacinar spaces, where CD31-positive vessels are seen (E, arrows).
Figure 2
Site-specific deposition of periostin-rich stroma on the invasive front of the activated stroma in PDAC parallels increased α-SMA expression of PSCs and decreased vascularity in the diseased pancreas. Compared with both normal parenchyma (A; original magnification, x50; white double-headed arrow) and organized ECM (white arrow), periostin expression dramatically increased at the interface where the activated stroma bordered on normal acini. The activation of stromal cells and detection of periostin expression in the CP-like changes surrounding the cancer occur even in areas where no cancer cells are visible. Although some periostin staining was detected in the interlobar septa (B and C; original magnification, x200), the strongest expression was detected in the periacinar spaces (B, arrows). Notice the encasement of acini by the periostin-rich ECMand emergence of tubular complexes (C, arrows; original magnification, x200). The percentage of CD31 (D) and α-SMA (E) staining in normal pancreas, CP, and PDAC tissues is graphically depicted. Results are expressed as mean ± SEM. Evaluation of CD31 (F, G, H) and α-SMA (I, J, K) using consecutive sections of normal (F, I), CP (G, J), and PDAC (H, K) tissues is shown.
Figure 3
Analysis of peritumoral expression of α-SMA, periostin, and CD31: Immunohistochemical analysis on PDAC tissue was performed using anti-α-SMA antibody with hematoxylin counterstaining. Notice the significantly higher number of PSCs compared with cancer cells (A; original magnification, x50). A demonstrative example of a highly desmoplastic PDAC is shown (B and C). Notice the mostly acellular ECM (white double arrow) and the increase in both α-SMA (B, black arrows) and CD31 (C, black arrows) staining in the peritumoral stroma. Colocalization of α-SMA (D; original magnification, x100), periostin (E), and CD31 (F) around cancer structures is demonstrated in sections without counterstaining.
Figure 4
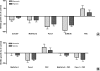
Effect of hypoxia on ECM protein synthesis and secretion of PSCs in vitro and quantification of secreted VEGF and endostatin in PCC and PSC SNs by ELISA: Sister clones of PSCs were kept under normoxic (N) and hypoxic (H) conditions for 1 (A) and 3 days (B) in serum-free medium. Matching CLs and SNs (SN) were analyzed by immunoblot analysis to evaluate the synthetic and secretory responses, respectively. All experiments were performed at least three times, and the results of densitometric analyses are presented as percent change (mean ± SEM) compared with the matching normoxic control (100%). Immunoblots were consecutively probed with α-SMA, periostin, type I collagen, fibronectin, and γ-tubulin antibodies. Sister clones of PCCs and PSCs were kept under normoxic and hypoxic conditions for 24 hours in serum-free medium. The amounts of VEGF (C) and endostatin (D) secreted in the SNs were quantified by ELISA. The values are expressed normalized to 100,000 cells. The experiments were performed at least three times using different PSC clones. *P = .037, **P = .0286.
Figure 5
Assessment of HUVEC growth after treatment with PCC and PSC SNs: HUVECs were seeded in 96-well plates (5000 cells per well) in complete endothelial cell growth medium (100 _µ_l per well). Twenty-four hours later, 100 _µ_l of cancer cell or stellate cell or coculture SN was added to the cells. Forty-eight hours later, cell growth was assessed by MTT assay corrected for day 0. The experiments were performed at least nine times using three different HUVEC clones and three different PSC clones. The effect of four different cancer cell lines and PSC SNs on HUVEC growth is shown in panel (A). The effect of the common SN after coculture of MiaPaCa-2 and Panc-1 with PSC is shown in panel (B). The growth-inhibiting or growth-promoting effects of SNs on HUVECs are shown as percent change of the control (0%).
Figure 6
Quantification of VEGF and endostatin secretion in pancreatic cancer-stellate cell coculture SNs by ELISA: PSC (200,000 cells per well) and PCC (400,000 cells per insert, 1-_µ_m pore size) were cocultured for 24 hours in serum-free medium. The amount of VEGF (A) and endostatin (C) secreted in the common SN was quantified by ELISA. The experiments were repeated at least twice using different PSC clones. In comparison to the mathematical sum of the individual controls (PCC + PSC), the increase of VEGF (B) and endostatin (D) in the common SN after coculture of MiaPaCa-2 and Panc-1 with PSCs is expressed in percentages.
Figure 7
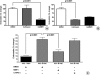
Quantification of secreted VEGF and endostatin by ELISA after exchange of SNs between PSCs and PCCs and assessment of MMP-12 and cathepsin B production by PSCs and PCCs: PSC (200,000 cells per well) and PCC (400,000 cells per well) were cultured in serum-free medium. After 24 hours, the individual SNs were exchanged and kept on the reciprocal cells for another day. PCC > PSC denotes cancer cell SN added to PSC; PSC > PCC denotes PSC SN added to cancer cells. In the control wells, serum-free medium was used to incubate the cells for 24 hours. The amount of VEGF (A) and endostatin (B) secreted was quantified by ELISA. In the control columns (PCC + PSC), the VEGF and endostatin produced by PCCs in 24 hours (black column) are mathematically added to the amount produced by the PSCs (white column above the black). The experiments were repeated three times using different PSC clones. Immunoblot analysis was used to measure the MMP-12 in the CLs and in the SN of cells (C). Five nanograms of MMP-12 was loaded to the first column as positive control (AG902; Millipore). MMP-12 secreted by cocultured PCC and PSC is shown (D). Secreted cathepsin B was quantified by ELISA (E). Error bars, SEM.
Figure 8
Quantification of secreted endostatin by ELISA after treatment with small-molecule broad-spectrum inhibitors of MMP and cathepsin: MiaPaCa-2 and Panc-1 (400,000 cells per well) were cultured alone (A) or cocultured with PSCs (C) for 24 hours in the presence of a small-molecule broad-spectrum MMP inhibitor (50 _µ_M), cathepsin inhibitor (10 _µ_M), or DMSO as control. To assess their effect on the cleavage of endostatin from the secreted collagen XVIII by cancer cells, inhibitors were added to the cancer cell SNs that were used to incubate PSC (200,000 cells per well) for 24 hours (B). Changes are expressed as percentages compared with the appropriate control (sum of the DMSO controls of PCC and PSC: PCC + PSC, 100%). PCC & PSC denotes the coculture of PCC and PSC in the presence of DMSO (control), small-molecule broad-spectrum MMP inhibitor (50 _µ_M), or cathepsin inhibitor (10 _µ_M). Error bars show the SEM. The experiments were repeated using five different PSC clones and two different cancer cell lines. The amount of endostatin secreted was quantified by ELISA.
Figure 9
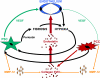
Schematic representation of the combined effects of cancer-stellate cell system on endothelial cells.
Similar articles
- Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity.
Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, Giese T, Büchler MW, Giese NA, Friess H. Erkan M, et al. Gastroenterology. 2007 Apr;132(4):1447-64. doi: 10.1053/j.gastro.2007.01.031. Epub 2007 Jan 25. Gastroenterology. 2007. PMID: 17408641 - Hypoxia enhances the interaction between pancreatic stellate cells and cancer cells via increased secretion of connective tissue growth factor.
Eguchi D, Ikenaga N, Ohuchida K, Kozono S, Cui L, Fujiwara K, Fujino M, Ohtsuka T, Mizumoto K, Tanaka M. Eguchi D, et al. J Surg Res. 2013 May;181(2):225-33. doi: 10.1016/j.jss.2012.06.051. Epub 2012 Jul 6. J Surg Res. 2013. PMID: 22795353 - Activated leukocyte cell adhesion molecule regulates the interaction between pancreatic cancer cells and stellate cells.
Zhang WW, Zhan SH, Geng CX, Sun X, Erkan M, Kleeff J, Xie XJ. Zhang WW, et al. Mol Med Rep. 2016 Oct;14(4):3627-33. doi: 10.3892/mmr.2016.5681. Epub 2016 Aug 26. Mol Med Rep. 2016. PMID: 27573419 Free PMC article. - Role of pancreatic stellate cells and periostin in pancreatic cancer progression.
Liu Y, Du L. Liu Y, et al. Tumour Biol. 2015 May;36(5):3171-7. doi: 10.1007/s13277-015-3386-2. Epub 2015 Apr 4. Tumour Biol. 2015. PMID: 25840689 Review. - Fibrotic Fortresses and Therapeutic Frontiers: Pancreatic Stellate Cells and the Extracellular Matrix in Pancreatic Cancer.
Sigirli S, Karakas D. Sigirli S, et al. Cancer Med. 2025 Jun;14(11):e70788. doi: 10.1002/cam4.70788. Cancer Med. 2025. PMID: 40437741 Free PMC article. Review.
Cited by
- [The microarchitecture of pancreatic cancer from the point of view of the pathologist and the radiologist].
Mayer P, Gaida MM. Mayer P, et al. Pathologe. 2021 Sep;42(5):524-529. doi: 10.1007/s00292-021-00949-2. Epub 2021 May 6. Pathologe. 2021. PMID: 33956172 Free PMC article. Review. German. - Therapeutic Strategies for Pancreatic-Cancer-Related Type 2 Diabetes Centered around Natural Products.
Park MN. Park MN. Int J Mol Sci. 2023 Nov 2;24(21):15906. doi: 10.3390/ijms242115906. Int J Mol Sci. 2023. PMID: 37958889 Free PMC article. Review. - Dual-Hit Strategy for Therapeutic Targeting of Pancreatic Cancer in Patient-Derived Xenograft Tumors.
Roy Chaudhuri T, Lin Q, Stachowiak EK, Rosario SR, Spernyak JA, Ma WW, Stachowiak MK, Greene MK, Quinn GP, McDade SS, Clynes M, Scott CJ, Straubinger RM. Roy Chaudhuri T, et al. Clin Cancer Res. 2024 Apr 1;30(7):1367-1381. doi: 10.1158/1078-0432.CCR-23-0131. Clin Cancer Res. 2024. PMID: 38270582 Free PMC article. - Soluble Compounds Released by Hypoxic Stroma Confer Invasive Properties to Pancreatic Ductal Adenocarcinoma.
Liu D, Steins A, Klaassen R, van der Zalm AP, Bennink RJ, van Tienhoven G, Besselink MG, Bijlsma MF, van Laarhoven HWM. Liu D, et al. Biomedicines. 2020 Oct 22;8(11):444. doi: 10.3390/biomedicines8110444. Biomedicines. 2020. PMID: 33105540 Free PMC article. - Pancreatic stellate cells - rising stars in pancreatic pathologies.
Hrabák P, Kalousová M, Krechler T, Zima T. Hrabák P, et al. Physiol Res. 2021 Dec 30;70(Suppl4):S597-S616. doi: 10.33549/physiolres.934783. Physiol Res. 2021. PMID: 35199546 Free PMC article. Review.
References
- Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, Kleeff J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6(10):1155–1161. - PubMed
- Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. - PubMed
- Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, Giese T, Buchler MW, Giese NA, Friess H. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–1464. - PubMed
- Reiser-Erkan C, Erkan M, Pan Z, Bekasi S, Giese NA, Streit S, Michalski CW, Friess H, Kleeff J. Hypoxia-inducible proto-oncogene Pim-1 is a prognostic marker in pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2008;7:1352–1359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous