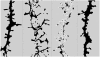Advances on the understanding of the origins of synaptic pathology in AD - PubMed (original) (raw)
Advances on the understanding of the origins of synaptic pathology in AD
Pascale Nathalie Lacor. Curr Genomics. 2007 Dec.
Abstract
Although Alzheimer's disease (AD) was first discovered a century ago, we are still facing a lack of definitive diagnosis during the patient's lifetime and are unable to prescribe a curative treatment. However, the past 10 years have seen a "revamping" of the main hypothesis about AD pathogenesis and the hope to foresee possible treatment. AD is no longer considered an irreversible disease. A major refinement of the classic beta-amyloid cascade describing amyloid fibrils as neurotoxins has been made to integrate the key scientific evidences demonstrating that the first pathological event occurring in AD early stages affects synaptic function and maintenance. A concept fully compatible with synapse loss being the best pathological correlate of AD rather than other described neuropathological hallmarks (amyloid plaques, neurofibrillary tangles or neuronal death). The notion that synaptic alterations might be reverted, thus offering a potential curability, was confirmed by immunotherapy experiments targeting beta-amyloid protein in transgenic AD mice in which cognitive functions were improved despite no reduction in the amyloid plaques burden. The updated amyloid cascade now integrates the synapse failure triggered by soluble Abeta-oligomers. Still no consensus has been reached on the most toxic Abeta conformations, neither on their site of production nor on their extra- versus intra-cellular actions. Evidence shows that soluble Abeta oligomers or ADDLs bind selectively to neurons at their synaptic loci, and trigger major changes in synapse composition and morphology, which ultimately leads to dendritic spine loss. However, the exact mechanism is not yet fully understood but is suspected to involve some membrane receptor(s).
Figures
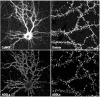
Fig. (1)
Upper: Neurons and more precisely dendritic spines were visualized on a cultured hippocampal neuron by labeling with anti-CaMKII and anti-drebrin antibodies. Lower: immunofluorescence for the ADDL bound onto the hippocampal cells was revealed using an anti-oligomer specific antibody. Labeling reveals that ADDL distributed along dendritic arbors particularly attack dendritic spines (as demonstrated by high degree of colocalization between drebrin and ADDLs).
Fig. (2)
ADDL binding to dendritic spines causes time-dependent changes in dendritic spine morphology and density as illustrated here by drebrin immunoreactivity, a dendritic spine marker. Computer-derived profile outlines were generated from a z-stack reconstruction of single dendritic branche imaged from a confocal scanning of drebrin-immunolabeled neurons. The treatment conditions represented here were in the following order ADDL 500nM for 5min, ADDL 500nM for 6hrs, ADDL 500nM for 24hrs and Vehicle for 24hrs. The same threshold setting was applied under the treatment conditions and show that both the dendritic spine density was decreased after ADDL treatment and that some spines are abnormally long after a prolonged ADDL treatment.
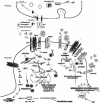
Fig. (3)
Summarized neurotoxic mechanisms of synaptically bound ADDLs. Proposed mechanistic pathway for synaptically-bound ADDLs implicated in the induction of aberrations in dendritic spine composition and morphology and how this mechanism is related to synaptic dysfunction and connectivity loss in AD. Aβ generated by cleavage of APP can accumulate and adopt oligomeric conformations of the ADDL-type that will bind to a postsynaptic membrane receptor (protein X) not yet identified but putatively located in the NMDA-R complex triggering Ca2+ influx followed by various changes in kinases/phosphatases activity that lead to: 1) reorganization of memory-essential receptors (NMDA-R, AMRA-R, EphB and InsR) from the cell surface most probably due to faulty receptor endocytosis. Various mechanisms have been proposed: one of them implicated Arc overexpression in the withdrawal of AMRA-R, while another suggested that NMDA-R endocytosis results from ADDL-induced activation of the a7nAchR and participation of signaling molecules such as STEP and Fyn. Alterations in dynamin 2 and endophilin, an enzyme involved in endocytic machinery controlling receptor turnover, has also been proposed. 2) deregulation of actin cytoskeletal dynamics which might result from the activation of Arc. At this level, rearrangement inside the actin-binding protein network, which is partially composed of Arc, Drebrin, Cofilin and Spinophilin, takes place disrupting the “spine morphology motor” and therefore proper synaptic plasticity. PAK and RhoGTPases activities are believed to play a major role in the actin cytoskeleton dynamics, its reduced activity leading to loss of drebrin and activation of cofilin, an actin-depolymerizing molecule. 3) Modifications of the microtubule network examplified by the hyperphosphorylation of tau and the alterations in tubulin and MAP2 (not represented here). 4) Possible interference with expression of survival and “killer” genes through the CREB pathway. For clarity purpose, other possible factors (e.g. oxidative stress, mitochondrial dysfunction, presynaptic neurotransmitter release) that might be implicated in synaptic receptor expression and spine morphology have been omitted.
Similar articles
- [Involvement of beta-amyloid in the etiology of Alzheimer's disease].
Tomiyama T. Tomiyama T. Brain Nerve. 2010 Jul;62(7):691-9. Brain Nerve. 2010. PMID: 20675873 Review. Japanese. - Is Alzheimer's disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid.
Nimmrich V, Ebert U. Nimmrich V, et al. Rev Neurosci. 2009;20(1):1-12. doi: 10.1515/revneuro.2009.20.1.1. Rev Neurosci. 2009. PMID: 19526730 Review. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - Urokinase-Type Plasminogen Activator Protects Cerebral Cortical Neurons from Soluble Aβ-Induced Synaptic Damage.
Diaz A, Merino P, Guo JD, Yepes MA, McCann P, Katta T, Tong EM, Torre E, Rangaraju S, Yepes M. Diaz A, et al. J Neurosci. 2020 May 20;40(21):4251-4263. doi: 10.1523/JNEUROSCI.2804-19.2020. Epub 2020 Apr 24. J Neurosci. 2020. PMID: 32332118 Free PMC article. - Drebrin in Alzheimer's Disease.
Ishizuka Y, Hanamura K. Ishizuka Y, et al. Adv Exp Med Biol. 2017;1006:203-223. doi: 10.1007/978-4-431-56550-5_12. Adv Exp Med Biol. 2017. PMID: 28865022 Review.
Cited by
- The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer's disease.
Ferreira ST, Klein WL. Ferreira ST, et al. Neurobiol Learn Mem. 2011 Nov;96(4):529-43. doi: 10.1016/j.nlm.2011.08.003. Epub 2011 Sep 6. Neurobiol Learn Mem. 2011. PMID: 21914486 Free PMC article. Review. - Aβ oligomer-induced synapse degeneration in Alzheimer's disease.
Wilcox KC, Lacor PN, Pitt J, Klein WL. Wilcox KC, et al. Cell Mol Neurobiol. 2011 Aug;31(6):939-48. doi: 10.1007/s10571-011-9691-4. Epub 2011 May 3. Cell Mol Neurobiol. 2011. PMID: 21538118 Free PMC article. Review. - The Role of ADF/Cofilin in Synaptic Physiology and Alzheimer's Disease.
Ben Zablah Y, Merovitch N, Jia Z. Ben Zablah Y, et al. Front Cell Dev Biol. 2020 Nov 12;8:594998. doi: 10.3389/fcell.2020.594998. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33282872 Free PMC article. Review. - Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5.
Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A. Renner M, et al. Neuron. 2010 Jun 10;66(5):739-54. doi: 10.1016/j.neuron.2010.04.029. Neuron. 2010. PMID: 20547131 Free PMC article. - Role of amyloid β protein receptors in mediating synaptic plasticity.
Li Y, Sun Z, Cao Q, Chen M, Luo H, Lin X, Xiao F. Li Y, et al. Biomed Rep. 2017 Apr;6(4):379-386. doi: 10.3892/br.2017.863. Epub 2017 Feb 21. Biomed Rep. 2017. PMID: 28413635 Free PMC article.
References
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch. Neurol. 2003;60:1119–1122. - PubMed
- Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer's disease. J. Alzheimers Dis. 2005;7:235–239. - PubMed
- Albert MS, Blacker D. Mild cognitive impairment and dementia. Annu. Rev. Clin. Psychol. 2006;2:379–388. - PubMed
- Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. Alzheimer's disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology. 1998;51:S2–17. - PubMed
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous