Dynamic partitioning of a glycosyl-phosphatidylinositol-anchored protein in glycosphingolipid-rich microdomains imaged by single-quantum dot tracking - PubMed (original) (raw)
Dynamic partitioning of a glycosyl-phosphatidylinositol-anchored protein in glycosphingolipid-rich microdomains imaged by single-quantum dot tracking
Fabien Pinaud et al. Traffic. 2009 Jun.
Abstract
Recent experimental developments have led to a revision of the classical fluid mosaic model proposed by Singer and Nicholson more than 35 years ago. In particular, it is now well established that lipids and proteins diffuse heterogeneously in cell plasma membranes. Their complex motion patterns reflect the dynamic structure and composition of the membrane itself, as well as the presence of the underlying cytoskeleton scaffold and that of the extracellular matrix. How the structural organization of plasma membranes influences the diffusion of individual proteins remains a challenging, yet central, question for cell signaling and its regulation. Here we have developed a raft-associated glycosyl-phosphatidyl-inositol-anchored avidin test probe (Av-GPI), whose diffusion patterns indirectly report on the structure and dynamics of putative raft microdomains in the membrane of HeLa cells. Labeling with quantum dots (qdots) allowed high-resolution and long-term tracking of individual Av-GPI and the classification of their various diffusive behaviors. Using dual-color total internal reflection fluorescence (TIRF) microscopy, we studied the correlation between the diffusion of individual Av-GPI and the location of glycosphingolipid GM1-rich microdomains and caveolae. We show that Av-GPI exhibit a fast and a slow diffusion regime in different membrane regions, and that slowing down of their diffusion is correlated with entry in GM1-rich microdomains located in close proximity to, but distinct, from caveolae. We further show that Av-GPI dynamically partition in and out of these microdomains in a cholesterol-dependent manner. Our results provide direct evidence that cholesterol-/sphingolipid-rich microdomains can compartmentalize the diffusion of GPI-anchored proteins in living cells and that the dynamic partitioning raft model appropriately describes the diffusive behavior of some raft-associated proteins across the plasma membrane.
Figures
Figure 1
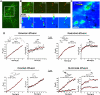
GPI-anchored avidin is targeted to the outer plasma membrane of HeLa cells and associates with lipid rafts as biochemically defined. (A) Schematic representation of the avidin/CD14 fusion (Av-GPI) construct. The full-length chicken avidin (amino acids 1-153) was fused in frame with the GPI-anchor sequence of CD14 (amino acids 318-376) to target Av-GPI to the outer membrane of HeLa cells. (B) Distribution of Av-GPI and GM1 in the membrane of HeLa cells with or without cross-linking with anti-avidin antibodies. Cross-linking induces the co-clustering of Av-GPI and GM1 (see also data at
http://fpinaud.bol.ucla.edu/index\_files/Traffic.htm
). Under the same conditions, endogenous transferrin receptors (TfR, non-raft proteins) remain evenly distributed (bottom row). Scale bars: 10 μm. (C) Av-GPI are enriched in detergent resistant membranes (DRM). After cold detergent extraction and sucrose gradient separation, most Av-GPI (96 %, see Table S1) are found in the light/DRM-rich fraction while the non-raft transferrin receptors (TfR, 97 %) are in the dense fraction. Cholesterol depletion with lovastatin induces a partial repartitioning of Av-GPI into the dense fraction (36 %) and a reduced recovery of Av-GPI (10 % of untreated). Lovastatin has no effect on the total protein recovery, the recovery of TfR or their distribution.
Figure 2
Single qdot tracking of Av-GPI by total internal reflection fluorescence (TIRF) microscopy, quantification and classification of diffusion modes. (A) First frame from a dual-color TIRF movie of a HeLa cell. Av-GPI in the ventral plasma membrane are labeled with qdots (red) and GM1 are labeled with Alexa-488 CT×B (green). (B) Selected frames from a region of interest (white square in (A)) in which diffusing Av-GPI are tracked. Diffusion trajectories are determined by the series of fitted positions, connected by a straight line. Notice that Alexa-488 CT×B bleaches fast compared to qdots and signal was nearly completely lost after 10 s (Movie M3). To facilitate visualization, the qdot PSF size was intentionally expanded. Tracking was performed on raw images. (C) Overlay of Av-GPI trajectories with the mean intensity projection image (ΣImean) for the Alexa-488 CT×B channel (see Materials and Methods). This approach allows colocalization studies of Av-GPI with fixed/slow diffusing GM1-rich domains despite the fast photobleaching of Alexa-488 CT×B (see data at
http://fpinaud.bol.ucla.edu/index\_files/Traffic.htm
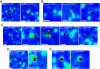
). (D) Analysis by probability distribution of square displacements (PDSD) for various trajectories and classification into diffusion modes. PDSD analysis was done on the first 10 % of time lags (t) (see Material and Methods). The resulting ri2(t) curves (black dots or square) were fitted with either pure Brownian, restricted or directed diffusion models (red, Table S2). When an Av-GPI experienced changes in diffusion during tracking, multiple ri2(t) curves were obtained and the mode of diffusion and the diffusion coefficient for each regime was determined. A representative sample of the various diffusion modes and diffusion coefficients of Av-GPI in HeLa cells are shown together with the corresponding trajectories and their duration. Notice that classification into various diffusion modes could not be accurately performed by simple visual inspection of the trajectories.
Figure 3
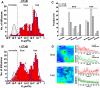
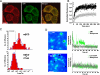
Bimodal diffusion of Av-GPI and interaction with GM1-rich microdomains. (A) Distribution of Av-GPI diffusion coefficients (red D-histogram) in HeLa cells without GM1 staining (-CT×B). Two Av-GPI diffusion regimes (fast and slow) are recovered after PDSD analysis, with D^fast = 3.8 10-2 μm2/s (SE 3.2-4.5 10-2 μm2/s, 55 %) and D^slow = 9.1 10-4 μm2/s (SE 0.7-1.2 10-3 μm2/s, 42 %). A D-histogram of qdots non-specifically bound to fibronectin (gray) is used to define immobile Av-GPI (3 %). The fraction of Av-GPI switching between fast and slow diffusion is 22 % (fraction determined using 1.3 10-2 μm2/s as a cut-off diffusion value to separate slow and fast diffusion. This value encompasses 95 % of the diffusion coefficients of the slow population). (B) Distribution of Av-GPI diffusion coefficients in the presence of the GM1-marker Alexa 488 cholera toxin-B (+CT×B). CT×B specifically induces a four-fold reduction in diffusion for the slow Av-GPI sub-population (D^slow+CT×B = 2.4 10-4 μm2/s, SE 1.7-3.4 10-4 μm2/s, 43 %). Fast Av-GPI are unaffected (D^fast+CT×B = 3.6 10-2 μm2/s, SE 2.9-4.6 10-2 μm2/s, 47 %). Arrows indicate the center of the distributions in the absence of CT×B in (A). (C) Effect of CT×B on the diffusion modes for slow and fast Av-GPI sub-populations. The increase in directed diffusions and stationary molecules at the expense of pure Brownian diffusion indicates that CT×B restrict the mobility of slow Av-GPI. For the fast diffusing sub-population, CT×B only induces a moderate reduction of Av-GPI with restricted diffusions. (D) Colocalization studies of Av-GPI with immobile/slow diffusing CT×B-labeled GM1 domains. A majority of slow Av-GPI (~70 %) are found colocalized with GM1-rich microdomains, while fast Av-GPI avoid these domains. Colocalization observed from ΣImean images are confirmed by correlating the fluorescence intensity time trace of qdot labeled Av-GPI (red) with the CT×B signal (green) along the diffusion trajectory. Because fluorescent signals varied from cell to cell, the background signal (grey) in close proximity to the trajectory is plotted for both red and green detection channels. Scale bars: 500 nm.
Figure 4
Dynamic partitioning of Av-GPI in and out of GM1-rich membrane microdomains. Example of Av-GPI exiting (A, B) or entering (C, D) cholera toxin B (CT×B) labeled GM1-rich microdomains (trajectory start: ▶ / stop: *). For Av-GPI exiting GM1-rich domains (A, B), initial signal colocalization and subsequent absence of colocalization between qdot and CT×B is observed from trajectory overlay on ΣImean images and from fluorescence intensity time traces. Domain exit correlates with an abrupt increase in the diffusion coefficient (instantaneous diffusions and PDSD analysis). Time periods where Av-GPI colocalize with GM1-rich domains are highlighted in green on instantaneous diffusion plots. The diffusion coefficients inside (Din) and outside (Dout) GM1-rich domains are determined from sub-trajectories MSD analysis and PDSD analysis (see Material and Methods). These diffusion values fall well within the distribution of diffusion coefficients for fast and slow Av-GPI determined in Fig. 3B. Detecting the entry of Av-GPI in GM1-rich domains (C, D) relies mainly on trajectory overlay with ΣImean images because labeled domains are usually bleached at the time of entry. Domain entry is associated with a clear reduction in diffusion coefficient. Notice that domains can be revisited (D). Some of these trajectories are from cells treated with lovastatin but are also representative of untreated cells since no difference in diffusive behaviors, interaction with GM1-rich domains or partitioning of Av-GPI were observed between both conditions. Scale bars: 500 nm.
Figure 5
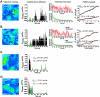
Imaging and tracking of Av-GPI and caveolae in HeLa cells. (A) Confocal images of HeLa cells expressing Av-GPI and Cav1-EGFP. Scale bar: 10 μm (B) Cross-linking of Av-GPI with anti-avidin antibodies induces the formation of Av-GPI and GM1-rich patches located in caveolae-rich regions of the membrane. Magnified region of interest (white squares) show that there is no extensive colocalization of these clusters with caveolar domains but that they are often contiguous (arrows). Scale bar: 5 μm. (C) First frame from a dual-color TIRF movie of a HeLa cell co-expressing Av-GPI (red) and Cav1-EGFP (green, left panel). After tracking, Av-GPI trajectories are overlaid on the mean intensity image of Cav1-EGFP (ΣImean) to detect interactions with caveolae (right panel). Scale bar: 3 μm. (D) Distribution of diffusion coefficients for Av-GPI (red) and caveolae (green). The two sub-populations of Av-GPI: fast (D^fastcav1−EGFP = 6.0 10-2 μm2/s, SE 5.3-6.7 10-2 μm2/s, 54 %) and slow (D^slowcav1−EGFP = 1.8 10-3 μm2/s, SE 0.9-3.5 10-3 μm2/s, 36 %) diffuse much faster than caveolae (D^cav = 7.7 10-5 μm2/s, SE 6.5-9.2 10-5 μm2/s), an indication that Av-GPI are rarely immobilized within caveolae.
Figure 6
Tracking of Av-GPI reveals rare colocalization with caveolae but slower diffusion in their proximity. (A) Fast Av-GPI diffuse mainly in caveolae-free part of the membrane. Colocalization with Cav1-EGFP labeled domains sometimes occurs but is not accompanied by apparent changes in diffusion. (B) Slow diffusing Av-GPI are often found adjacent to caveolae. (C) Examples of Av-GPI undergoing changes in diffusion during tracking. As in (B), intervals of slow diffusion (white arrows) often localize in close proximity to caveolae. (D) On rare occasions, changes in diffusion upon direct interaction with caveolae are observed. Slow diffusion domains colocalizing with caveolae are highlighted in red and indicated by white arrows. (E) Slow Av-GPI colocalized with caveolae have diffusion coefficients that may or may not be similar to that of the caveola itself, suggesting immobilization or simple confinement within the caveolar invagination (see also Fig. S6). Scale bars (A-E): 1 μm.
Figure 7
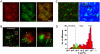
Acute cholesterol depletion by mβCD induces a slowing down of Av-GPI and an apparent re-distribution in non-caveolar GM1-rich microdomains. (A) Three dimensional projection of live HeLa cells treated with mβCD for 1 hr. Acute cholesterol depletion leads to the rounding up of cells, decreased Av-GPI surface density and redistribution of Av-GPI and GM1 into colocalizing punctuated domains in the membrane. Scale bar: 15 μm. (B) FRAP of membrane Av-GPI directly after mβCD treatment (open circle) and after cholesterol replenishment (black circle). The diffusion of Av-GPI is reduced after treatment with mβCD but can be restored to pre-treatment levels after re-incubation into serum-supplemented media for 20 hr. Both FRAP curves are averaged over three cells and acquired at 37 °C. (C) Distribution of Av-GPI diffusion coefficients after mβCD treatment (top panel) and mβCD treatment followed by imaging in the presence of cholera toxin B sub-unit (+CT×B, bottom panel). A single diffusing population of Av-GPI was recovered (D^mβCD∕−CT×B = 3.5 10-4 μm2/s, SE 2.5-4.9 10-4 μm2/s). The addition of CT×B induces a ~80-fold decrease in diffusion and nearly complete immobilization of Av-GPI (D^mβCD∕+CT×B = 2.7 10-5 μm2/s, SE 2.2-3.3 10-5 μm2/s). (D) Correlation between Av-GPI trajectories and GM1-rich domains (top panel) or Cav1-EGFP domains (bottom panel) in mβCD-treated HeLa cells. About 50 % of Av-GPI colocalized with slow/immobile CT×B-labeled GM1-rich microdomains. Fluorescent CT×B signal along the trajectory of Av-GPI indicates that Av-GPI interact directly with these domains. After mβCD-treatment Av-GPI diffused in domains adjacent to but distinct from caveolae, as verified by the absence of Cav1-EGFP fluorescent signal along the trajectory of the AV-GPI (marked by an asterisk). Scale bars: 1 μm (top) and 2 μm (bottom).
Similar articles
- Oligodendrocytes direct glycosyl phosphatidylinositol-anchored proteins to the myelin sheath in glycosphingolipid-rich complexes.
Krämer EM, Koch T, Niehaus A, Trotter J. Krämer EM, et al. J Biol Chem. 1997 Apr 4;272(14):8937-45. doi: 10.1074/jbc.272.14.8937. J Biol Chem. 1997. PMID: 9083015 - Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidylinositol (GPI)-anchored proteins in epithelial cells.
Lipardi C, Mora R, Colomer V, Paladino S, Nitsch L, Rodriguez-Boulan E, Zurzolo C. Lipardi C, et al. J Cell Biol. 1998 Feb 9;140(3):617-26. doi: 10.1083/jcb.140.3.617. J Cell Biol. 1998. PMID: 9456321 Free PMC article. - Glycosphingolipid and Glycosylphosphatidylinositol Affect Each Other in and on the Cell.
Guo Z. Guo Z. Chembiochem. 2023 Jul 3;24(13):e202200761. doi: 10.1002/cbic.202200761. Epub 2023 Jun 1. Chembiochem. 2023. PMID: 36935354 Free PMC article. Review. - GPI-anchored protein organization and dynamics at the cell surface.
Saha S, Anilkumar AA, Mayor S. Saha S, et al. J Lipid Res. 2016 Feb;57(2):159-75. doi: 10.1194/jlr.R062885. Epub 2015 Sep 22. J Lipid Res. 2016. PMID: 26394904 Free PMC article. Review.
Cited by
- Tracking Single Molecules in Biomembranes: Is Seeing Always Believing?
Yu Y, Li M, Yu Y. Yu Y, et al. ACS Nano. 2019 Oct 22;13(10):10860-10868. doi: 10.1021/acsnano.9b07445. Epub 2019 Oct 7. ACS Nano. 2019. PMID: 31589406 Free PMC article. Review. - Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation.
Li X, Wang X, Yang Y, Li R, He Q, Fang X, Luu DT, Maurel C, Lin J. Li X, et al. Plant Cell. 2011 Oct;23(10):3780-97. doi: 10.1105/tpc.111.091454. Epub 2011 Oct 18. Plant Cell. 2011. PMID: 22010034 Free PMC article. - Tracking single proteins in live cells using single-chain antibody fragment-fluorescent quantum dot affinity pair.
Iyer G, Michalet X, Chang YP, Weiss S. Iyer G, et al. Methods Enzymol. 2010;475:61-79. doi: 10.1016/S0076-6879(10)75003-5. Methods Enzymol. 2010. PMID: 20627153 Free PMC article. - Probing cellular events, one quantum dot at a time.
Pinaud F, Clarke S, Sittner A, Dahan M. Pinaud F, et al. Nat Methods. 2010 Apr;7(4):275-85. doi: 10.1038/nmeth.1444. Epub 2010 Mar 30. Nat Methods. 2010. PMID: 20354518 Review. - Lipid rafts and detergent-resistant membranes in epithelial keratinocytes.
McGuinn KP, Mahoney MG. McGuinn KP, et al. Methods Mol Biol. 2014;1195:133-44. doi: 10.1007/7651_2014_71. Methods Mol Biol. 2014. PMID: 24504930 Free PMC article.
References
- Singer S, Nicolson G. The Fluid Mosaic Model of the Structure of Cell Membranes Cell Membranes Are Viewed as 2 Dimensional Solutions of Oriented Globular Proteins and Lipids. Science. 1972;175(23):720–731. - PubMed
- Ryan TA, Myers J, Holowka D, Baird B, Webb WW. Molecular crowding on the cell surface. Science. 1988;239:61–64. - PubMed
- Edidin M. The state of lipid rafts: From model membranes to cells. Annual Review of Biophysics and Biomolecular Structure. 2003;32:257–283. - PubMed
- Simons K, Vaz WLC. Model systems, lipid rafts, and cell membranes. Ann Rev Biophys Biomol Struct. 2004;33:269–295. - PubMed
- Kusumi A, Koyama-Honda I, Suzuki K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic. 2004;5(4):213–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources