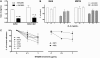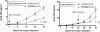Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma - PubMed (original) (raw)
. 2009 Jul 9;114(2):371-9.
doi: 10.1182/blood-2008-11-191577. Epub 2009 May 5.
Pierfrancesco Tassone, Teru Hideshima, Sonia Vallet, Puru Nanjappa, Seth A Ettenberg, Zhenxin Shen, Nipun Patel, Yu-Tzu Tai, Dharminder Chauhan, Constantine Mitsiades, Rao Prabhala, Noopur Raje, Kenneth C Anderson, David R Stover, Nikhil C Munshi
Affiliations
- PMID: 19417213
- PMCID: PMC2714212
- DOI: 10.1182/blood-2008-11-191577
Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma
Mariateresa Fulciniti et al. Blood. 2009.
Abstract
Decreased activity of osteoblasts (OBs) contributes to osteolytic lesions in multiple myeloma (MM). The production of the soluble Wnt inhibitor Dickkopf-1 (DKK1) by MM cells inhibits OB activity, and its serum level correlates with focal bone lesions in MM. Therefore, we have evaluated bone anabolic effects of a DKK1 neutralizing antibody (BHQ880) in MM. In vitro BHQ880 increased OB differentiation, neutralized the negative effect of MM cells on osteoblastogenesis, and reduced IL-6 secretion. In a severe combined immunodeficiency (SCID)-hu murine model of human MM, BHQ880 treatment led to a significant increase in OB number, serum human osteocalcin level, and trabecular bone. Although BHQ880 had no direct effect on MM cell growth, it significantly inhibited growth of MM cells in the presence of bone marrow stromal cells (BMSCs) in vitro. This effect was associated with inhibition of BMSC/MM cell adhesion and production of IL-6. In addition, BHQ880 up-regulated beta-catenin level while down-regulating nuclear factor-kappaB (NF-kappaB) activity in BMSC. Interestingly, we also observed in vivo inhibition of MM cell growth by BHQ880 treatment in the SCID-hu murine model. These results confirm DKK1 as an important therapeutic target in myeloma and provide the rationale for clinical evaluation of BHQ880 to improve bone disease and to inhibit MM growth.
Figures
Figure 1
BHQ880 reverses the inhibitory effect of MM cells on osteoblastogenesis. (A) BMSC obtained from BM aspirate of MM patients were stimulated with OB differentiation media for 3 weeks in the presence of IgG1 isotype control antibody or 1 μg/mL BHQ880 and in the absence (top panel) or in the presence (bottom panel) of INA-6 MM cells. At the end of the culture period, the cells were fixed in 10% formaldehyde and stained with Alizarin Red for 30 minutes. (B) Calcium deposition was quantified in these cultures using osteogenesis assay kit. The amount of calcium deposits in differentiated BMSC treated with BHQ880 (red column) is expressed as percent of calcium deposition compared with differentiated BMSC treated with isotype control antibody (blue column) in the absence and presence of MM cells. (C) Supernatants from these assays were analyzed for huIL-6 level by ELISA. IL-6 production was suppressed after treatment with BHQ880 in supernatants from both cultures with and without MM cells.
Figure 2
BHQ880 overcomes the growth promoting effect of BMSC on both IL-6-dependent and IL-6-independent MM cells. (A) INA-6 MM cells were cultured without and with BMSC in the presence of different doses of BHQ880 for 24 to 48 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and is presented as percentage change from control. Data represent mean ± SD of 4 independent experiments performed in triplicates. (B) MM1S, OPM1, OPM2, U266, XG1 MM cell lines, and (C) primary cells from 5 MM patients were cultured without (−) and with (+) BMSC in the presence of BHQ880 for 48 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and presented as change from control cells cultured in the absence of BMSC (−). Data represent mean ± SD of 4 independent experiments performed in triplicate.
Figure 3
BHQ880 inhibits IL-6 production and MM cell adhesion to BMSC from MM patients. (A) Both BMSC from MM patient alone and cocultured with INA-6 cells were treated with isotype control antibody (□) or 1 μg/mL BHQ880 (■) for 24 hours. Culture supernatant was then analyzed for huIL-6 level by ELISA. (B) INA-6 and MM1S MM cells were cultured in the presence of BMSC and increasing concentrations of rIL-6 (0, 0.5, and 1 ng/mL) with and without BHQ880 for 24 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and is presented as percentage change from control. (C) Serum-starved MM cell lines and CD138+ patient MM cells were labeled with calcein AM, washed, and added to BMSC-coated plates for 4 hours with isotype control or increasing amounts of BHQ880 (0.1 and 1 μg/mL). Adhesion was evaluated by measuring the absorbance using 492/520 nm filter set with a fluorescence plate reader. Data represent mean ± SD of 4 independent experiments performed in triplicate.
Figure 4
BHQ880 inhibits IL-6 production and MM cell adhesion and modulates β-catenin and NF-κB pathways in BMSC from MM patients. BMSC were incubated for 24 hours with 1 μg/mL isotype control or BHQ880. Cells were harvested and subjected to cell lysis. (A) Cytoplasmic fraction was subjected to immunoblotting using anti-phospho β-catenin antibody and anti–β-catenin antibody. (B) Nuclear fraction was subjected to EMSA for NF-κB binding activity. Oct-1 DNA binding activity by EMSA was used as internal control. (C) Nuclear proteins (15 μg) were analyzed for NF-κB activity using the Transcription Factor ELISA kit, which measures DNA binding activity. Absorbance was obtained with a spectrophotometer at 450 nm and is presented as optical density (OD).
Figure 5
BHQ880 improves bone disease in a murine model of human MM. SCID-hu mice were injected with INA-6 cells into the implanted bone and treated with isotype control (n = 7) or BHQ880 (n = 7) after first detection of tumor. (A) After injection of INA-6 MM cells directly into the human bone implant, serum from SCID-hu mice were monitored for production of DKK1 (blue column) and shuIL-6R (green column) by ELISA. (B) One month after treatment, bone chips were retrieved and decalcified, and sections from control and BHQ880-treated bones were immunohistochemically stained for ALP (top), and the number of OB was measured per field in isotype control and BHQ880-treated bones (bottom). Sections were observed and photographed with a Nikon transmitted light microscope. Original magnification ×200. Data are expressed as ± SD. (C) Serum level of human osteocalcin in murine blood was evaluated at the end of the treatment period by ELISA. Data are expressed as mean ± SD. (D) Sections from control and BHQ880-treated bones were stained by H&E showing increased bone tissue and decreased MM cell number in the BHQ880-treated compared with isotype control-treated samples. Sections were observed and photographed with a Nikon transmitted light microscope. Original magnification ×100.
Figure 6
BHQ880 inhibits myeloma cell growth in SCID-hu mice. (A) In the early treatment model, mice were injected with isotype control (200 μg per mouse 3×/week; n = 7) and BHQ880 (200 μg per mouse 3×/week; n = 7) at the first detection of tumor. Serum samples were collected weekly, and the level of shuIL-6R was measured by ELISA. (B) In the late treatment model, mice were injected with isotype control (200 μg per mouse 3×/week; n = 3) and BHQ880 (200 μg per mouse 3×/week; n = 3) 3 weeks after first detection of tumor. Serum samples were collected weekly, and the level of shuIL-6R was measured by ELISA. Baseline values before treatment were not significantly different among groups.
Similar articles
- Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo.
Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr. Yaccoby S, et al. Blood. 2007 Mar 1;109(5):2106-11. doi: 10.1182/blood-2006-09-047712. Epub 2006 Oct 26. Blood. 2007. PMID: 17068150 Free PMC article. - Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma.
Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD Jr, Evans HR, Snowden JA, Stover DR, Vanderkerken K, Croucher PI. Heath DJ, et al. J Bone Miner Res. 2009 Mar;24(3):425-36. doi: 10.1359/jbmr.081104. J Bone Miner Res. 2009. PMID: 19016584 - A Phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events.
Iyer SP, Beck JT, Stewart AK, Shah J, Kelly KR, Isaacs R, Bilic S, Sen S, Munshi NC. Iyer SP, et al. Br J Haematol. 2014 Nov;167(3):366-75. doi: 10.1111/bjh.13056. Epub 2014 Aug 19. Br J Haematol. 2014. PMID: 25139740 Clinical Trial. - Dickkopf-1 is a key regulator of myeloma bone disease: opportunities and challenges for therapeutic intervention.
Zhou F, Meng S, Song H, Claret FX. Zhou F, et al. Blood Rev. 2013 Nov;27(6):261-7. doi: 10.1016/j.blre.2013.08.002. Epub 2013 Sep 2. Blood Rev. 2013. PMID: 24054128 Free PMC article. Review. - Dickkopf-1: a suitable target for the management of myeloma bone disease.
Gavriatopoulou M, Dimopoulos MA, Christoulas D, Migkou M, Iakovaki M, Gkotzamanidou M, Terpos E. Gavriatopoulou M, et al. Expert Opin Ther Targets. 2009 Jul;13(7):839-48. doi: 10.1517/14728220903025770. Expert Opin Ther Targets. 2009. PMID: 19530987 Review.
Cited by
- Targeting Wnt pathways in disease.
Zimmerman ZF, Moon RT, Chien AJ. Zimmerman ZF, et al. Cold Spring Harb Perspect Biol. 2012 Nov 1;4(11):a008086. doi: 10.1101/cshperspect.a008086. Cold Spring Harb Perspect Biol. 2012. PMID: 23001988 Free PMC article. Review. - Model of translational cancer research in multiple myeloma.
Yasui H, Ishida T, Maruyama R, Nojima M, Ikeda H, Suzuki H, Hayashi T, Shinomura Y, Imai K. Yasui H, et al. Cancer Sci. 2012 Nov;103(11):1907-12. doi: 10.1111/j.1349-7006.2012.02384.x. Epub 2012 Aug 17. Cancer Sci. 2012. PMID: 22809142 Free PMC article. Review. - Novel therapeutic targets in myeloma bone disease.
Webb SL, Edwards CM. Webb SL, et al. Br J Pharmacol. 2014 Aug;171(16):3765-76. doi: 10.1111/bph.12742. Br J Pharmacol. 2014. PMID: 24750110 Free PMC article. Review. - Mesenchymal stem cells in multiple myeloma: a therapeutical tool or target?
Xu S, De Veirman K, De Becker A, Vanderkerken K, Van Riet I. Xu S, et al. Leukemia. 2018 Jul;32(7):1500-1514. doi: 10.1038/s41375-018-0061-9. Epub 2018 Feb 22. Leukemia. 2018. PMID: 29535427 Free PMC article. Review. - High serum levels of Dickkopf-1 are associated with a poor prognosis in prostate cancer patients.
Rachner TD, Thiele S, Göbel A, Browne A, Fuessel S, Erdmann K, Wirth MP, Fröhner M, Todenhöfer T, Muders MH, Kieslinger M, Rauner M, Hofbauer LC. Rachner TD, et al. BMC Cancer. 2014 Sep 2;14:649. doi: 10.1186/1471-2407-14-649. BMC Cancer. 2014. PMID: 25182503 Free PMC article.
References
- Roodman GD. Pathogenesis of myeloma bone disease. Blood Cells Mol Dis. 2004;32:290–292. - PubMed
- Sezer O. Myeloma bone disease. Hematology. 2005;10(suppl 1):19–24. - PubMed
- Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–4935. - PubMed
- Vanderkerken K, De Leenheer E, Shipman C, et al. Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res. 2003;63:287–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01-78378/PHS HHS/United States
- R01-124929/PHS HHS/United States
- P01 CA078378/CA/NCI NIH HHS/United States
- P01 CA155258/CA/NCI NIH HHS/United States
- P50 CA100707/CA/NCI NIH HHS/United States
- P50-100007/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous