MT1-MMP and type II collagen specify skeletal stem cells and their bone and cartilage progeny - PubMed (original) (raw)
MT1-MMP and type II collagen specify skeletal stem cells and their bone and cartilage progeny
Ludmila Szabova et al. J Bone Miner Res. 2009 Nov.
Abstract
Skeletal formation is dependent on timely recruitment of skeletal stem cells and their ensuing synthesis and remodeling of the major fibrillar collagens, type I collagen and type II collagen, in bone and cartilage tissues during development and postnatal growth. Loss of the major collagenolytic activity associated with the membrane-type 1 matrix metalloproteinase (MT1-MMP) results in disrupted skeletal development and growth in both cartilage and bone, where MT1-MMP is required for pericellular collagen dissolution. We show here that reconstitution of MT1-MMP activity in the type II collagen-expressing cells of the skeleton rescues not only diminished chondrocyte proliferation, but surprisingly, also results in amelioration of the severe skeletal dysplasia associated with MT1-MMP deficiency through enhanced bone formation. Consistent with this increased bone formation, type II collagen was identified in bone cells and skeletal stem/progenitor cells of wildtype mice. Moreover, bone marrow stromal cells isolated from mice expressing MT1-MMP under the control of the type II collagen promoter in an MT1-MMP-deficient background showed enhanced bone formation in vitro and in vivo compared with cells derived from nontransgenic MT1-MMP-deficient littermates. These observations show that type II collagen is not stringently confined to the chondrocyte but is expressed in skeletal stem/progenitor cells (able to regenerate bone, cartilage, myelosupportive stroma, marrow adipocytes) and in the chondrogenic and osteogenic lineage progeny where collagenolytic activity is a requisite for proper cell and tissue function.
Figures
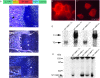
FIG. 1
Transgene construction and expression of MT1-MMP in skeletal tissues. (A) Linear diagram of the collagen II/MT1-MMP transgene used for expression of MT1-MMP in cartilage tissue. Briefly, the mouse MT1-MMP cDNA (red) was placed under control of the type II collagen promoter/enhancer (green). Also shown is the β-globin intron (yellow) and the SV40 poly A signal sequence (blue). (B) Rat chondrosarcoma cells transfected with the transgene construct show abundant membrane-associated immunoreactivity when reacted with MT1-MMP–specific antibodies. Compare with C, depicting cells transfected with empty vector. (D) Northern blot of total RNA isolated from neonate mice reacted with an MT1-MMP–specific probe detects the endogenous transcript “E” and the transgene derived transcript “TG.” Lane 1, MT1-MMP−/−;T+ mouse; lane 2, MT1-MMP+/−;T+ littermate; lane 3, MT1-MMP−/−;T+ littermate; lane 4, MT1-MMP+/+ littermate. (E) H&E-stained femur from neonate control mouse showing the primary ossification center (asterisk) and the prospective epiphyseal growth plates “GP” of the distal condyle. (F) Dark field in situ hybridization image from the same block reacted with an _mt1-mmp_–specific antisense probe. Note the signal in periosteum (arrows) and the primary spongiosa (arrowheads). Signal is also detected in the proliferation zone of the prospective growth plate, although to a lesser extent than elsewhere (asterisk). (G) Section from femur of an MT1-MMP−/−;T+ mouse reacted with an antisense mt1-mmp probe. Note that expression is now restricted predominantly to the cartilage tissue (asterisks), whereas less, but still detectable, signal is found in the ossifying tissue (arrows inset in G, showing periosteal “P” and marrow signal between trabecular bone “TB”). (H) Immunoblot of costal chondrocyte extracts from neonate mice detecting MT1-MMP. Lane 1, MT1-MMP−/−;T+ mouse; lane 2, MT1-MMP−/− mouse; lane 3, MT1-MM+/−;T+ littermate; lane 4, MT1-MMP+/− littermate; lane 5, MT1-MMP+/+ littermate. Scale bars: (B and C) 10 μm; (E–G) 100 μm.
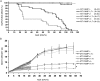
FIG. 2
Survival and weight gain in mice. (A) Survival of mice. Note the significant reduction in mortality associated with expression of the MT1-MMP transgene (T+) in the MT1-MMP–deficient background (MT1-MMP−/−;T+) compared with MT1-MMP−/− mice. (B) Weight gain in mice after birth measured daily until 21 days of age and then weekly. The expression of the MT1-MMP transgene in MT1-MMP–deficient mice significantly increases weight gain over nontransgenic littermates. _a_Statistical significance (p < 0.05).
FIG. 3
Cartilage-specific expression of MT1-MMP is associated with increased chondrocyte proliferation and longitudinal bone growth. (A) Alizarin red/Alcian blue–stained femora from 8-day-old littermates showing the difference in bone growth. Actual length of the femora was established using X-ray analysis (data not shown). (B) MT1-MMP−/−;T+ mice show significantly increased bone length compared with nontransgenic MT1-MMP−/− littermates. (C–F) Increased bone length in the MT1-MMP−/−;T+ mice is correlated with higher BrdU incorporation in chondrocytes of the epiphyseal growth plate (D) compared with MT1-MMP−/− mice (C). (E) MT1-MMP+/− control littermate. (F) Enumeration of proliferating cells from MT1-MMP−/−, MT1-MMP−/−;T+, and MT1-MMP+/− mice. Scale bar: (C–E): 100 μm. _a_Statistical significance (p < 0.01).
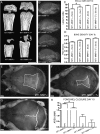
FIG. 4
Expression of collagen II driven MT1-MMP is associated with increased bone formation. (A) CT images of femora from 15-day-old littermates. Note that presence of the MT1-MMP transgene in MT1-MMP−/−;T+ mice increases the length of bone and the degree of ossification. (B) Crania from 8-day-old mice show increased skull length in MT1-MMP−/−;T+ mice (bottom) compared with nontransgenic MT1-MMP−/− littermates (second from bottom). (C and D) Quantitation of skull length and tissue mineral density (TMD) based on the data shown in A and B. (E–G) Alizarin red/Alcian blue whole mount preparation of crania from 8-day-old mice visualizing the posterior fontanelle. MT1-MMP−/− mice (E) show significantly larger fontanelle sizes compared with the MT1-MMP−/−;T+ littermates (F). However, virtually complete closure as seen in MT1-MMP+/− control is not achieved (G). (H) Measurements of fontanelle areas in 8-day-old mice. Note the expression of the MT1-MMP transgene confers significant reduction of the fontanelle size. Scale bars: (B) 5 mm; (A and E–G): 1 mm. _a_Statistical significance in C and H (p < 0.001).
FIG. 5
Type II collagen in bone cells and function of MT1-MMP. (A) In situ hybridization of the parietal bone from a wildtype mouse. Bone cells (arrowheads) and osteocytes (arrow) react with a collagen (II) α1–specific antisense probe. (B) Section serial to that shown in A reacted with collagen (II) α1 sense control probe. (C and D) Immunohistochemistry for collagen II in bone shows that bone cells and soft connective tissue stain positive for type II collagen. Inset in C depicts high-power magnification of the bone outline in the boxed area with type II collagen–positive osteoblasts (arrowheads) and osteocytes (arrows). (D) Negative control of nonserial section from the same specimen. (E) Sagittal section through the tibial cortex of a 59-day-old wildtype mouse showing abundant cortical bone and associated marrow. (F) Equivalent section of MT1-MMP–deficient littermate showing abundant scarring and fibrosis of the periosteum (above the dashed line) including ectopic calcification (asterisk) and highly aberrant bone matrix below the dashed line. Several osteogenic cells are entrapped in the fibrotic tissue (arrows) (G) Cartoon depicting the result of a cellular deficit in MT1-MMP–dependent collagenolysis and the consequences for remodeling of pericellular collagen. In the absence of MT1-MMP–mediated collagen remodeling, cells become entrapped in collagen as shown in F (arrows), and bone formation and periosteal maintenance are perturbed. Scale bar: (A–F) 100 μm.
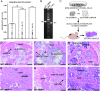
FIG. 6
Expression of type II collagen in BMSCs and analysis of their osteogenic potential. (A) BMSCs derived from either MT1-MMP−/−, MT1-MMP−/−;T+, or MT1-MMP+/− mice show an increasing ability to form in vitro mineralized matrix in osteoconductive medium when measured as alizarin red retention. (B) In accordance with the results observed by in situ hybridization, immunostaining, and in the mineralization assay, BMSCs from wildtype mice show MT1-MMP and type II collagen–specific mRNA by RT-PCR (samples run on the same gel, but not adjacent lanes). This observation explains the expression derived from the collagen II–MT1-MMP transgene and the resulting MT1-MMP expression in BMSCs isolated from MT1-MMP−/−;T+ mice. (C) To test whether the osteogenic potential observed in vitro is biologically relevant, BMSCs on osteoconductive media were implanted in nude mice and allowed to form ectopic ossicles. (D and E) Cells derived from wildtype mice showed the ability to form both bone and marrow in hydroxyapatite (D) or Gelfoam scaffolds (E). Cells derived from MT1-MMP−/− mice failed almost completely to form bone in either hydroxyapatite (F) or Gelfoam (G) osteoinductive carriers nor did they support formation of marrow. Instead, extensive fibrosis was observed (arrows) (F and G). When MT1-MMP−/− cells expressed the collagen II–driven MT1-MMP transgene, the osteogenic potential was restored, and the cells generated abundant bone and marrow with both hydroxyapatite (H) and Gelfoam (I) as scaffolds, thus showing that the type II collagen promoter is active in BMSCs. Scale bar: (D–I) 100 μm. _a_Statistical significance (p < 0.001).
Similar articles
- MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth.
Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H. Holmbeck K, et al. J Cell Biol. 2003 Nov 10;163(3):661-71. doi: 10.1083/jcb.200307061. J Cell Biol. 2003. PMID: 14610065 Free PMC article. - Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis.
Shi J, Son MY, Yamada S, Szabova L, Kahan S, Chrysovergis K, Wolf L, Surmak A, Holmbeck K. Shi J, et al. Dev Biol. 2008 Jan 1;313(1):196-209. doi: 10.1016/j.ydbio.2007.10.017. Epub 2007 Oct 23. Dev Biol. 2008. PMID: 18022611 Free PMC article. - MT1-MMP: a tethered collagenase.
Holmbeck K, Bianco P, Yamada S, Birkedal-Hansen H. Holmbeck K, et al. J Cell Physiol. 2004 Jul;200(1):11-9. doi: 10.1002/jcp.20065. J Cell Physiol. 2004. PMID: 15137053 Review. - Skeletal stem cells.
Bianco P, Robey PG. Bianco P, et al. Development. 2015 Mar 15;142(6):1023-7. doi: 10.1242/dev.102210. Development. 2015. PMID: 25758217 Free PMC article. Review.
Cited by
- Running on time: the role of circadian clocks in the musculoskeletal system.
Dudek M, Meng QJ. Dudek M, et al. Biochem J. 2014 Oct 1;463(1):1-8. doi: 10.1042/BJ20140700. Biochem J. 2014. PMID: 25195734 Free PMC article. - Tracking the Cartoon mouse phenotype: Hemopexin domain-dependent regulation of MT1-MMP pericellular collagenolytic activity.
Sakr M, Li XY, Sabeh F, Feinberg TY, Tesmer JJG, Tang Y, Weiss SJ. Sakr M, et al. J Biol Chem. 2018 May 25;293(21):8113-8127. doi: 10.1074/jbc.RA117.001503. Epub 2018 Apr 11. J Biol Chem. 2018. PMID: 29643184 Free PMC article. - Site-1 protease regulates skeletal stem cell population and osteogenic differentiation in mice.
Patra D, DeLassus E, Mueller J, Abou-Ezzi G, Sandell LJ. Patra D, et al. Biol Open. 2018 Feb 22;7(2):bio032094. doi: 10.1242/bio.032094. Biol Open. 2018. PMID: 29437042 Free PMC article. - Scaffold-Free Fabrication of Osteoinductive Cellular Constructs Using Mouse Gingiva-Derived Induced Pluripotent Stem Cells.
Okawa H, Kayashima H, Sasaki J, Miura J, Kamano Y, Kosaka Y, Imazato S, Yatani H, Matsumoto T, Egusa H. Okawa H, et al. Stem Cells Int. 2016;2016:6240794. doi: 10.1155/2016/6240794. Epub 2016 Mar 27. Stem Cells Int. 2016. PMID: 27110251 Free PMC article. - Control of craniofacial development by the collagen receptor, discoidin domain receptor 2.
Mohamed FF, Ge C, Hallett SA, Bancroft AC, Cowling RT, Ono N, Binrayes AA, Greenberg B, Levi B, Kaartinen VM, Franceschi RT. Mohamed FF, et al. Elife. 2023 Jan 19;12:e77257. doi: 10.7554/eLife.77257. Elife. 2023. PMID: 36656123 Free PMC article.
References
- van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. - PubMed
- Ricard-Blum S, Ruggiero F. The collagen superfamily: From the extracellular matrix to the cell membrane. Pathol Biol (Paris) 2005;53:430–442. - PubMed
- Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. - PubMed
- Goto T, Yamaza T, Tanaka T. Cathepsins in the osteoclast. J Electron Microsc (Tokyo) 2003;52:551–558. - PubMed
- Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113:377–381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases