Myosin-interacting guanine exchange factor (MyoGEF) regulates the invasion activity of MDA-MB-231 breast cancer cells through activation of RhoA and RhoC - PubMed (original) (raw)
Myosin-interacting guanine exchange factor (MyoGEF) regulates the invasion activity of MDA-MB-231 breast cancer cells through activation of RhoA and RhoC
D Wu et al. Oncogene. 2009.
Abstract
The small guanine triphosphatase (GTPase) proteins RhoA and RhoC are essential for tumor invasion and/or metastasis in breast carcinomas. However, it is poorly understood how RhoA and RhoC are activated in breast cancer cells. Here we describe the role of myosin-interacting guanine nucleotide exchange factor (Myo-GEF) in regulating RhoA and RhoC activation as well as cell polarity and invasion in an invasive breast cancer cell line MDA-MB-231. RNA-interference (RNAi)-mediated depletion of MyoGEF in MDA-MB-231 cells not only suppresses the activation of RhoA and RhoC, but also decreases cell polarity and invasion activity. The dominant-negative mutants of RhoA and RhoC, but not Rac1 and Cdc42, dramatically decrease actin polymerization induced by MyoGEF. In addition, MyoGEF co-localizes with nonmuscle myosin IIA (NMIIA) to the front of migrating cells, and depletion of NMIIA by RNAi disrupts the polarized localization of MyoGEF at the cell leading edge, suggesting a role for NMIIA in regulating MyoGEF localization and function. Moreover, MyoGEFprotein levels significantly increase in infiltrating ductal carcinomas as well as in invasive breast cancer cell lines. Taken together, our results suggest that MyoGEF cooperates with NMIIA to regulate the polarity and invasion activity of breast cancer cells through activation of RhoA and RhoC.
Figures
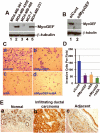
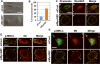
Figure 1. MyoGEF is required for the invasion activity of MDA-MB-231 cells
(A) Immunoblot analysis with anti-MyoGEF antibody shows that MyoGEF is expressed in MDA-MB-231 and MDA-MB-435S cells, but not in MDA-MB-361, MDA-MB-468, and MCF-7 cells. (B) Immunoblot analysis confirms the depletion of MyoGEF in MDA-MB-231 cells by RNAi. (C) MDA-MB-231 cells depleted of MyoGEF and/or NMIIA were subjected to Matrigel invasion assays. (D) Images in (C) were quantitated by using the NIH ImageJ program. (E) Immunohistochemical analysis of a breast cancer tissue array with MyoGEF antibody. Three arrays were analyzed independently and similar results were obtained. Immunohistochemistry with preimmune serum shows light, background straining (data not shown). Images in (C) and (E) were taken by using a 20x objective (Leica DMI 6000 B microscope).
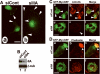
Figure 2. Depletion of MyoGEF represses RhoA and RhoC activation in MDA-MB-231 cells
(A) Immunoblot analysis confirms the depletion of MyoGEF in MDA-MB-231 cells by RNAi. (B) The image in (A) was quantitated by using the NIH ImageJ program to estimate the efficiency of MyoGEF depletion in MDA-MB-231 cells by RNAi. (C-F) Depletion of MyoGEF decreases the amount of active RhoA (C) and RhoC (D), but not Rac1 (E) and CDc42 (F), in MDA-MB-231 cells. ~6% of transfected cell lysates were used as control to estimate the amount of total RhoA, RhoC, Rac1, and Cdc42. (G) The images in (C), (D), (E), and (F) were quantitated by using the NIH ImageJ program.
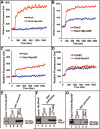
Figure 3. In vitro activation of RhoA, RhoC, and Rac1, but not Cdc42, by MyoGEF
(A-D) The immunoprecipitated Myc-MyoGEF from transfected HeLa cells could activate RhoA (A), RhoC( B), and Rac1 (C), but not Cdc42 (D) in a fluorescence-based GEF assay. (E) ThioHis-MyoGEF (full-length) could bind both GDP-RhoA (lane 4) and GTP-RhoA (lane 5). (F) A MyoGEF fragment (amino acids 71-388) that contain the DH domain could bind both GDP-RhoC (lane 4) and GTP-RhoC (lane 5). (G) ThioHis-MyoGEF could bind GTP-Rac1 (lane 5) but not GDP-Rac1 (lane 4). D, preloaded with GDP; T, preloaded with GTP.
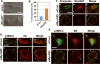
Figure 4. MyoGEF colocalizes with actin-myosin filaments at the cell leading edge
(A) MDA-MB-231 cells were subjected to immunofluorescence with anti-MyoGEF antibody (green) and rhodaminephalloidin (red). (B) Immunoblot analysis of total cell lysates from MDA-MB-231 with anti-MyoGEF antibody. Note that a single band was recognized by MyoGEF antibody in MDA-MB-231 cell lysates. (C) Exogenously expressed GFP-MyoGEF (green) colocalizes with actin filaments (red) in transfected MDA-MB-231 cells. (D) Exogenously expressed GFP-IIA (green) colocalizes with endogenous MyoGEF (red) at the cell leading edge of transfected MDA-MB-231 cells. (E) Exogenously expressed GFP-MyoGEF (green) colocalizes with endogenous NMIIA (red) at the cell leading edge of transfected MDA-MB-231 cells. Bars, 10 μm.
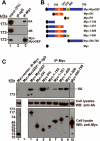
Figure 5. MyoGEF interacts with NMIIA
(A) MDA-MB-231 cells expressing Myc-MyoGEF were subjected to immunoprecipitation with anti-Myc antibody followed by immunoblot analysis with anti-IIA or anti-IIB antibodies. Note that Myc-MyoGEF binds to NMIIA but not NMIIB. (B) Schematic diagram of MyoGEF fragments that were used in (C). (C) Interactions between Myc-tagged MyoGEF fragments and endogenous NMIIA. Full-length MyoGEF (lane 3) as well as MyoGEF fragments Myc-PH (lane 5), Myc-1-409 (lane 8), and Myc-1-500 (lane 9) could pull down a significant amount of endogenous NMIIA. Note that cell lysate from lane 3 was also used for immunoprecipitation with normal IgG (lane 2). ~5% of cell lysates were loaded.
Figure 6. Depletion of MyoGEF by RNAi impairs MDA-MB-231 cell polarity
(A) MDA-MB-231 cells treated with control siRNA (siCont) or MyoGEF siRNA (siMyoGEF) for 48 h were trypsinized, replated on fibronectin-coated coverslips, and cultured for an additional 6 h. Note that cells depleted of MyoGEF did not polarize. (B) Quantitation of nonpolarized MDA-MB-231 cells treated with control or MyoGEF siRNAs. (C) MDA-MB-231 cells treated with siCont or siMyoGEF were subjected to immunofluorescence with MyoGEF antibody (red) and FITC-phalloidin (green). (D) MDA-MB-231 cells treated with siCont or siMyoGEF were stained with antibodies specific for p-MRLC (green) and NMIIA (red). (E) MDA-MB-231 cells treated with siCont or siMyoGEF were stained with antibodies specific for p-MRLC (green) and NMIIB (red). Bar in (A), 80 μm; Bars in (C), (D), and (E), 10 μm
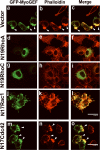
Figure 7. NMIIA is required for polarized localization of MyoGEF as well as the formation of MyoGEF-induced actin bundles
(A) MDA-MB-231 cells treated with control siRNA (siCont; panel a) or NMIIA siRNA (siIIA; panel b) were subjected to immunofluorescence with anti-MyoGEF antibody. (B) MDA-MB-231 cells treated with siCont or siIIA were subjected to immunoblot analysis with antibodies specific for NMIIA or β-tubulin. (C-D) A plasmid encoding GFP-MyoGEF was cotransfected into HeLa cells with siCont or siIIA. The transfected cells were subjected to immunofluorescence with anti-IIA antibody (C) or phalloidin (D). Bars, 10 μm.
Figure 8. Expression of dominant negative mutants N19RhoA and N19RhoC inhibits the formation of MyoGEF-induced actin bundles
A plasmid encoding GFP-MyoGEF was cotransfected into HeLa cells with an empty vector (a-c) or plasmids encoding N19RhoA (d-f), N19RhoC (g-i), N17Rac1 (j-l), or N17Cdc42 (m-o). Note that co-transfection of N19RhoA or N19RhoC decreases the formation of massive actin bundles induced by GFP-MyoGEF. Bars, 60 μm.
Similar articles
- Rho isoform-specific interaction with IQGAP1 promotes breast cancer cell proliferation and migration.
Casteel DE, Turner S, Schwappacher R, Rangaswami H, Su-Yuo J, Zhuang S, Boss GR, Pilz RB. Casteel DE, et al. J Biol Chem. 2012 Nov 2;287(45):38367-78. doi: 10.1074/jbc.M112.377499. Epub 2012 Sep 19. J Biol Chem. 2012. PMID: 22992742 Free PMC article. - RhoA and RhoC have distinct roles in migration and invasion by acting through different targets.
Vega FM, Fruhwirth G, Ng T, Ridley AJ. Vega FM, et al. J Cell Biol. 2011 May 16;193(4):655-65. doi: 10.1083/jcb.201011038. J Cell Biol. 2011. PMID: 21576392 Free PMC article. - RhoA, RhoB and RhoC have different roles in cancer cell migration.
Ridley AJ. Ridley AJ. J Microsc. 2013 Sep;251(3):242-9. doi: 10.1111/jmi.12025. Epub 2013 Mar 12. J Microsc. 2013. PMID: 23488932 Review. - The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.
Boissier P, Huynh-Do U. Boissier P, et al. Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
Cited by
- Endothelial RhoA GTPase is essential for in vitro endothelial functions but dispensable for physiological in vivo angiogenesis.
Zahra FT, Sajib MS, Ichiyama Y, Akwii RG, Tullar PE, Cobos C, Minchew SA, Doçi CL, Zheng Y, Kubota Y, Gutkind JS, Mikelis CM. Zahra FT, et al. Sci Rep. 2019 Aug 12;9(1):11666. doi: 10.1038/s41598-019-48053-z. Sci Rep. 2019. PMID: 31406143 Free PMC article. - The Cytotoxicity of Carbon Nanotubes and Hydroxyapatite, and Graphene and Hydroxyapatite Nanocomposites against Breast Cancer Cells.
Nguyen T, Maniyar A, Sarkar M, Sarkar TR, Neelgund GM. Nguyen T, et al. Nanomaterials (Basel). 2023 Jan 30;13(3):556. doi: 10.3390/nano13030556. Nanomaterials (Basel). 2023. PMID: 36770518 Free PMC article. - FARP1 boosts CDC42 activity from integrin αvβ5 signaling and correlates with poor prognosis of advanced gastric cancer.
Hirano T, Shinsato Y, Tanabe K, Higa N, Kamil M, Kawahara K, Yamamoto M, Minami K, Shimokawa M, Arigami T, Yanagita S, Matushita D, Uenosono Y, Ishigami S, Kijima Y, Maemura K, Kitazono I, Tanimoto A, Furukawa T, Natsugoe S. Hirano T, et al. Oncogenesis. 2020 Feb 6;9(2):13. doi: 10.1038/s41389-020-0190-7. Oncogenesis. 2020. PMID: 32029704 Free PMC article. - Hyaluronan-CD44 interaction promotes c-Src-mediated twist signaling, microRNA-10b expression, and RhoA/RhoC up-regulation, leading to Rho-kinase-associated cytoskeleton activation and breast tumor cell invasion.
Bourguignon LY, Wong G, Earle C, Krueger K, Spevak CC. Bourguignon LY, et al. J Biol Chem. 2010 Nov 19;285(47):36721-35. doi: 10.1074/jbc.M110.162305. Epub 2010 Sep 15. J Biol Chem. 2010. PMID: 20843787 Free PMC article.
References
- Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, et al. Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem. 2000;275:10141–9. - PubMed
- Ahram M, Sameni M, Qiu RG, Linebaugh B, Kirn D, Sloane BF. Rac1-induced endocytosis is associated with intracellular proteolysis during migration through a three-dimensional matrix. Exp Cell Res. 2000;260:292–303. - PubMed
- Arthur WT, Ellerbroek SM, Der CJ, Burridge K, Wennerberg K. XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J Biol Chem. 2002;277:42964–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K22 HL071542/HL/NHLBI NIH HHS/United States
- P20 RR015563/RR/NCRR NIH HHS/United States
- P20 RR 015563/RR/NCRR NIH HHS/United States
- K22 HL 071542/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous