Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice - PubMed (original) (raw)
Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice
J-G Wang et al. J Thromb Haemost. 2009 Jul.
Abstract
Background: Tissue factor (TF) is present in blood in various forms, including small membrane vesicles called microparticles (MPs). Elevated levels of these MPs appear to play a role in the pathogenesis of thrombosis in a variety of diseases, including sepsis.
Objective: Measure levels of MP TF activity and activation of coagulation in control and endotoxemic mice.
Materials and methods: MPs were prepared from plasma by centrifugation. The procoagulant activity (PCA) of MPs was measured using a two-stage chromogenic assay. We also measured levels of thrombin-antithrombin and the number of MPs.
Results: Lipopolysaccharide (LPS) increased MP PCA in wild-type mice; this PCA was significantly reduced by an anti-mouse TF antibody (1H1) but not with an anti-human TF antibody (HTF-1). Conversely, in mice expressing only human TF, MP PCA was inhibited by HTF-1 but not 1H1. MPs from wild-type mice had 6-fold higher levels of PCA using mouse factor (F)VIIa compared with human FVIIa, which is consistent with reported species-specific differences in FVIIa. Mice expressing low levels of human TF had significantly lower levels of MP TF activity and TAT than mice expressing high levels of human TF; however, there were similar levels of phosphatidylserine (PS)-positive MPs. Importantly, levels of MP TF activity in wild-type mice correlated with levels of TAT but not with PS-positive MPs in endotoxemic mice.
Conclusion: These results suggest that the levels of TF-positive MPs can be used as a biomarker for evaluating the risk of disseminated intravascular coagulation in endotoxemia.
Conflict of interest statement
Disclosure and Conflict of Interests
The authors state they have no conflict of interest.
Figures
Fig. 1
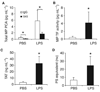
Lipopolysaccharide (LPS) increases levels of microparticles (MP), MP procoagulant activity (PCA) and TAT in wild-type mice. Wild-type C57/B6J mice were given an intraperitoneal injection of either phosphate-buffered saline (PBS) or LPS and blood was collected after 6 h. (A) Total MP PCA was measured using mouse factor (F)VIIa and human FX in the presence of rat IgG (white bars) or 1H1 (black bars). (B) MP tissue factor (TF) activity was calculated by subtraction of the MP PCA generated in the presence of 1H1 from the total MP PCA generated in the presence of IgG control. (C) Levels of TAT in the plasma from mice treated with PBS (white bar) or LPS treated (black bar). (D) The number of PS-positive MPs in plasma from mice treated with PBS (white bar) or LPS (right bar) is shown. All results are shown as mean ± SD, n = 4–6 mice per group. Asterisks indicate statistically significant differences between the groups.
Fig. 2
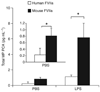
Measurement of microparticle (MP) procoagulant activity (PCA) in wild-type mice using mouse or human factor (F)VIIa. Total MP PCA in plasma from wild-type mice treated with either phosphate-buffered saline (PBS) control (left bars) or lipopolysaccharide (LPS) (right bars) was measured using either human FVIIa (white bars) or mouse FVIIa (black bars) and human FX. The inset is an enhanced plot of the PBS control. Data are shown as mean ± SD (n = 5). Asterisks indicate a statistically significant difference between the MP PCA observed with human FVIIa.
Fig. 3
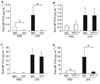
Species-specific inhibition of microparticle (MP) procoagulant activity (PCA) from wild-type and human TF (HTF) mice. (A and B) Total MP PCA in plasma from wild-type mice treated with either phosphate-buffered saline (PBS) control (left bars) or lipopolysaccharide (LPS) (right bars) was measured using human factor (F)VIIa and human FX in the presence of 1H1 or isotype IgG or HTF-1 or isotype IgG. Data are shown as mean ± SD (n = 6–7). (C and D) Total MP PCA in plasma from HTF mice treated with either PBS control (left bars) or LPS (right bars) was measured using human FVIIa and human FX in the presence of 1H1 or isotype IgG or HTF-1 or isotype IgG. Data are shown as mean ± SD (n = 4). Asterisks indicate statistically significant differences between the groups.
Fig. 4
Effect of human factor (F)VIIai and annexin V on microparticle (MP) procoagulant activity (PCA) from wild-type and human TF (HTF) mice. (A) Total PCA in MPs isolated from plasma of wild-type mice treated with either phosphate-buffered saline (PBS) control (white bar) or lipopolysaccharide (LPS) (black bars) was measured using mouse FVIIa and human FX in the presence of 1H1, isotype IgG, human FVIIai or annexin V. (B) Total MP PCA from plasma of HTF mice treated with either PBS control (white bar) or LPS (black bars) was measured using human FVIIa and human FX in the presence of HTF-1, isotype IgG, human FVIIai or annexin V. Data are shown as mean ± SD (n = 4). Asterisks indicate statistically significant differences relative to the IgG control.
Fig. 5
Levels of microparticle (MP) procoagulant activity (PCA), MP tissue factor (TF) activity, TAT and MPs in human TF (HTF) and Low TF Mice. Total MP PCA (A) and MP TF activity (B) in plasma from HTF and low mice treated with either PBS control (white bars) or LPS (black bars) was measured using human FVIIa and human FX. TF activity in the MPs was determined using HTF-1. Data are shown as mean ± SD (n = 4–5). (C) Levels of TAT in the plasma from HTF (left bars) and low TF (right bars) mice treated with phosphate-buffered saline (PBS) (white bars) or lipopolysaccharide (LPS)-treated (black bars). (D) Number of PS-positive MPs in plasma from HTF mice (left bars) and low TF mice (right bars). All results are shown as mean ± SD, (n = 4–6). Asterisks indicate statistically significant differences between the groups.
Fig. 6
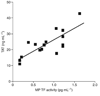
Levels of microparticle (MP) tissue factor (TF) activity correlate with levels of TAT. A significant linear correlation was observed between levels of MP TF activity and TAT in endotoxemic wild-type mice (R = 0.78, P < 0.01, n = 16).
Similar articles
- Procoagulant microparticles promote coagulation in a factor XI-dependent manner in human endotoxemia.
Mooberry MJ, Bradford R, Hobl EL, Lin FC, Jilma B, Key NS. Mooberry MJ, et al. J Thromb Haemost. 2016 May;14(5):1031-42. doi: 10.1111/jth.13285. Epub 2016 Apr 5. J Thromb Haemost. 2016. PMID: 26857798 Free PMC article. - Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice.
Pawlinski R, Wang JG, Owens AP 3rd, Williams J, Antoniak S, Tencati M, Luther T, Rowley JW, Low EN, Weyrich AS, Mackman N. Pawlinski R, et al. Blood. 2010 Aug 5;116(5):806-14. doi: 10.1182/blood-2009-12-259267. Epub 2010 Apr 21. Blood. 2010. PMID: 20410508 Free PMC article. - Procoagulant microparticles in dogs with immune-mediated hemolytic anemia.
Kidd L, Geddings J, Hisada Y, Sueda M, Concannon T, Nichols T, Merricks E, Mackman N. Kidd L, et al. J Vet Intern Med. 2015 May-Jun;29(3):908-16. doi: 10.1111/jvim.12583. Epub 2015 Apr 13. J Vet Intern Med. 2015. PMID: 25871966 Free PMC article. - Measurement of microparticle tissue factor activity in clinical samples: A summary of two tissue factor-dependent FXa generation assays.
Hisada Y, Alexander W, Kasthuri R, Voorhees P, Mobarrez F, Taylor A, McNamara C, Wallen H, Witkowski M, Key NS, Rauch U, Mackman N. Hisada Y, et al. Thromb Res. 2016 Mar;139:90-7. doi: 10.1016/j.thromres.2016.01.011. Epub 2016 Jan 18. Thromb Res. 2016. PMID: 26916302 Free PMC article. Review. - Platelet microparticles and vascular cells interactions: a checkpoint between the haemostatic and thrombotic responses.
Morel O, Morel N, Freyssinet JM, Toti F. Morel O, et al. Platelets. 2008 Feb;19(1):9-23. doi: 10.1080/09537100701817232. Platelets. 2008. PMID: 18231934 Review.
Cited by
- Caspase-1-mediated pathway promotes generation of thromboinflammatory microparticles.
Rothmeier AS, Marchese P, Petrich BG, Furlan-Freguia C, Ginsberg MH, Ruggeri ZM, Ruf W. Rothmeier AS, et al. J Clin Invest. 2015 Apr;125(4):1471-84. doi: 10.1172/JCI79329. Epub 2015 Feb 23. J Clin Invest. 2015. PMID: 25705884 Free PMC article. - Extracellular vesicles participate in the pathogenesis of sepsis.
Tian C, Wang K, Zhao M, Cong S, Di X, Li R. Tian C, et al. Front Cell Infect Microbiol. 2022 Dec 12;12:1018692. doi: 10.3389/fcimb.2022.1018692. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36579343 Free PMC article. Review. - Plasma TF activity predicts cardiovascular mortality in patients with acute myocardial infarction.
Steppich BA, Braun SL, Stein A, Demetz G, Groha P, Schömig A, von Beckerath N, Kastrati A, Ott I. Steppich BA, et al. Thromb J. 2009 Jul 2;7:11. doi: 10.1186/1477-9560-7-11. Thromb J. 2009. PMID: 19570241 Free PMC article. - Sepsis-associated disseminated intravascular coagulation and thromboembolic disease.
Semeraro N, Ammollo CT, Semeraro F, Colucci M. Semeraro N, et al. Mediterr J Hematol Infect Dis. 2010 Aug 13;2(3):e2010024. doi: 10.4084/MJHID.2010.024. Mediterr J Hematol Infect Dis. 2010. PMID: 21415977 Free PMC article. - Monocytic microparticles activate endothelial cells in an IL-1β-dependent manner.
Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP, Mackman N. Wang JG, et al. Blood. 2011 Aug 25;118(8):2366-74. doi: 10.1182/blood-2011-01-330878. Epub 2011 Jun 23. Blood. 2011. PMID: 21700772 Free PMC article.
References
- Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. - PubMed
- Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nature Med. 2003;9:458–462. - PubMed
- Bruggemann LW, Drijfhout JW, Reitsma PH, Spek CA. Alternatively spliced tissue factor in mice: induction by Streptococcus pneumoniae. J Thromb Haemost. 2006;4:918–920. - PubMed
- Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, Freyssinet JM. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–2604. - PubMed
- Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PGM, ten Have K, Eijsman L, Hack CE, Sturk A. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation. 1997;96:3534–3541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous