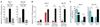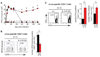IL-21 is required to control chronic viral infection - PubMed (original) (raw)
IL-21 is required to control chronic viral infection
Heidi Elsaesser et al. Science. 2009.
Erratum in
- Science. 2009 Aug 21;325(5943):946
Abstract
CD4+ and CD8+ T cell functions are rapidly aborted during chronic infection, preventing viral clearance. CD4+ T cell help is required throughout chronic infection so as to sustain CD8+ T cell responses; however, the necessary factor(s) provided by CD4+ T cells are currently unknown. Using a mouse model of chronic viral infection, we demonstrated that interleukin-21 (IL-21) is an essential component of CD4+ T cell help. In the absence of IL-21 signaling, despite elevated CD4+ T cell responses, CD8+ T cell responses are severely impaired. CD8+ T cells directly require IL-21 to avoid deletion, maintain immunity, and resolve persistent infection. Thus, IL-21 specifically sustains CD8+ T cell effector activity and provides a mechanism of CD4+ T cell help during chronic viral infection.
Figures
Fig. 1
IL-21 mRNA expression during viral infection. IL-21 mRNA expression was quantified by real-time reverse transcription polymerase chain reaction after LCMV-Arm or LCMV–Cl 13 infection in (A) uninfected (gray bar), LCMV–Cl 13 infected (black bar), or LCMV–Cl 13 infected and CD4+ T cell–depleted (striped bar) splenocytes or in (B) the indicated sorted cell population. Red Bars indicate cells sorted from LCMV-Arm–infected animals; black bars indicate cells sorted from LCMV–Cl 13–infected animals. (C) Frequency of IFN-γ+ SMARTA T cells that simultaneously produced IL-2 or IL-21 on day 9 and day 30 after infection. Error bars represent the average ± SD of 3 to 5 mice per group and 2 to 4 independent experiments. *P < 0.05, **P = 0.4.
Fig. 2
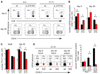
IL-21 sustains virus-specific CD8+ T cells during viral persistence. (A) LCMV-GP33–41 MHC class I tetramer staining on days 9 or 30 after LCMV-Arm or LCMV–Cl 13 infection. Numbers indicate the frequency of tetramer+ CD8+ T cells in WT and _Il21r_−/− (KO) mice. (B) Number of tetramer+ CD8+ T cells on days 9 and 30 after LCMV-Arm or LCMV–Cl 13 infection of WT and _Il21r_−/− mice. (C) Number of WT or _Il21r_−/− P14 T cells after infection of Il21r+/+ mice. (D) Frequency of WT and _Il21r_−/− LCMV-GP33–41 tetramer+ CD8+ T cells in bone-marrow chimeric mice on days 8 and 28 in the blood and on day 28 in the spleen after LCMV–Cl 13 infection. Numbers in parentheses indicate the total proportion of WT and _Il21r_−/− CD8+ T cells. Bar graphs represent the average ± SD of 3 to 5 mice per group and represent 2 to 4 independent experiments. *P<0.05.
Fig. 3
IL-21 suppresses CD4+ T cell function. (A) LCMV-GP61–80–specific CD4+ T cell responses on days 9 and 30 after LCMV-Arm or LCMV–Cl 13 infection of WT or _Il21r_−/− mice. Numbers in each quadrant indicate the frequency of IFN-γ+ and IL-2+ CD4+ T cells and the numbers in parentheses indicate the frequency of IFN-γ and IL-2 double-positive cells. (B) The frequency and number of CD4+ T cells that simultaneously produce IL-2 and IFN-γ on days 9 or 30 after LCMV-Arm or LCMV–Cl 13 infection. (C) Frequency of LCMV-GP61–80 tetramer+ CD4+ T on day 30 after LCMV-Arm or LCMV–Cl 13 infection. The bar graph represents the number of tetramer+ CD4+ T cells. Bar graphs summarize the average ± SD of 3 to 4 mice per group and 3 to 4 independent experiments. *P < 0.05.
Fig. 4
IL-21 is required to purge chronic viral infection. (A) Serum viral titers after LCMV-Arm (triangles) or LCMV–Cl 13 (circles) infection of WT (black) or _Il21r_−/− (red) mice. Data are expressed as plaque-forming units (PFU) per milliliter of serum. The dashed line indicates the lower limit of detection (200 PFU/ml). *P < 0.05 for LCMV–Cl 13 infection of WT versus _Il21r_−/− mice. (B and C) The frequency and number of (B) LCMV-GP33–41 tetramer+ CD8+ T cells and (C) IFN-γ+ CD4+ T cells on day 100 after LCMV–Cl 13 infection of WT and _Il21r_−/− mice. Time points and bar graphs represent the average ± SD of 3 to 4 mice per group and 3 independent experiments. *P < 0.05.
Comment in
- Immunology. A chronic need for IL-21.
Johnson LD, Jameson SC. Johnson LD, et al. Science. 2009 Jun 19;324(5934):1525-6. doi: 10.1126/science.1176487. Science. 2009. PMID: 19541985 No abstract available.
Similar articles
- A vital role for interleukin-21 in the control of a chronic viral infection.
Yi JS, Du M, Zajac AJ. Yi JS, et al. Science. 2009 Jun 19;324(5934):1572-6. doi: 10.1126/science.1175194. Epub 2009 May 14. Science. 2009. PMID: 19443735 Free PMC article. - CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection.
Matloubian M, Concepcion RJ, Ahmed R. Matloubian M, et al. J Virol. 1994 Dec;68(12):8056-63. doi: 10.1128/JVI.68.12.8056-8063.1994. J Virol. 1994. PMID: 7966595 Free PMC article. - A little 'help' from IL-21 during persistent viral infection.
McGavern DB. McGavern DB. J Mol Cell Biol. 2010 Feb;2(1):8-10. doi: 10.1093/jmcb/mjp021. Epub 2009 Oct 1. J Mol Cell Biol. 2010. PMID: 19797314 Free PMC article. - CD8 T cell dysfunction during chronic viral infection.
Shin H, Wherry EJ. Shin H, et al. Curr Opin Immunol. 2007 Aug;19(4):408-15. doi: 10.1016/j.coi.2007.06.004. Epub 2007 Jul 25. Curr Opin Immunol. 2007. PMID: 17656078 Review. - Induction and maintenance of CD8+ T cells specific for persistent viruses.
van Leeuwen EM, ten Berge IJ, van Lier RA. van Leeuwen EM, et al. Adv Exp Med Biol. 2007;590:121-37. doi: 10.1007/978-0-387-34814-8_9. Adv Exp Med Biol. 2007. PMID: 17191382 Review. No abstract available.
Cited by
- Clonal lineage tracing reveals mechanisms skewing CD8+ T cell fate decisions in chronic infection.
Kasmani MY, Zander R, Chung HK, Chen Y, Khatun A, Damo M, Topchyan P, Johnson KE, Levashova D, Burns R, Lorenz UM, Tarakanova VL, Joshi NS, Kaech SM, Cui W. Kasmani MY, et al. J Exp Med. 2023 Jan 2;220(1):e20220679. doi: 10.1084/jem.20220679. Epub 2022 Oct 31. J Exp Med. 2023. PMID: 36315049 Free PMC article. - Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection.
Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Aubert RD, et al. Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21182-7. doi: 10.1073/pnas.1118450109. Epub 2011 Dec 12. Proc Natl Acad Sci U S A. 2011. PMID: 22160724 Free PMC article. - The CD4⁺ T-cell help signal is transmitted from APC to CD8⁺ T-cells via CD27-CD70 interactions.
Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. Feau S, et al. Nat Commun. 2012 Jul 10;3:948. doi: 10.1038/ncomms1948. Nat Commun. 2012. PMID: 22781761 Free PMC article. - Harnessing CD4⁺ T cell responses in HIV vaccine development.
Streeck H, D'Souza MP, Littman DR, Crotty S. Streeck H, et al. Nat Med. 2013 Feb;19(2):143-9. doi: 10.1038/nm.3054. Epub 2013 Feb 6. Nat Med. 2013. PMID: 23389614 Free PMC article. Review. - HCV-Specific T Cell Responses During and After Chronic HCV Infection.
Luxenburger H, Neumann-Haefelin C, Thimme R, Boettler T. Luxenburger H, et al. Viruses. 2018 Nov 17;10(11):645. doi: 10.3390/v10110645. Viruses. 2018. PMID: 30453612 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI085043/AI/NIAID NIH HHS/United States
- R21 AI077012/AI/NIAID NIH HHS/United States
- AI082975/AI/NIAID NIH HHS/United States
- AI077012/AI/NIAID NIH HHS/United States
- U01 AI082975/AI/NIAID NIH HHS/United States
- AI070845/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous