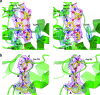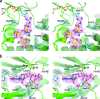The crystal structures of substrate and nucleotide complexes of Enterococcus faecium aminoglycoside-2''-phosphotransferase-IIa [APH(2'')-IIa] provide insights into substrate selectivity in the APH(2'') subfamily - PubMed (original) (raw)
The crystal structures of substrate and nucleotide complexes of Enterococcus faecium aminoglycoside-2''-phosphotransferase-IIa [APH(2'')-IIa] provide insights into substrate selectivity in the APH(2'') subfamily
Paul G Young et al. J Bacteriol. 2009 Jul.
Abstract
Aminoglycoside-2''-phosphotransferase-IIa [APH(2'')-IIa] is one of a number of homologous bacterial enzymes responsible for the deactivation of the aminoglycoside family of antibiotics and is thus a major component in bacterial resistance to these compounds. APH(2'')-IIa produces resistance to several clinically important aminoglycosides (including kanamycin and gentamicin) in both gram-positive and gram-negative bacteria, most notably in Enterococcus species. We have determined the structures of two complexes of APH(2'')-IIa, the binary gentamicin complex and a ternary complex containing adenosine-5'-(beta,gamma-methylene)triphosphate (AMPPCP) and streptomycin. This is the first crystal structure of a member of the APH(2'') family of aminoglycoside phosphotransferases. The structure of the gentamicin-APH(2'')-IIa complex was solved by multiwavelength anomalous diffraction methods from a single selenomethionine-substituted crystal and was refined to a crystallographic R factor of 0.210 (R(free), 0.271) at a resolution of 2.5 A. The structure of the AMPPCP-streptomycin complex was solved by molecular replacement using the gentamicin-APH(2'')-IIa complex as the starting model. The enzyme has a two-domain structure with the substrate binding site located in a cleft in the C-terminal domain. Gentamicin binding is facilitated by a number of conserved acidic residues lining the binding cleft, with the A and B rings of the substrate forming the majority of the interactions. The inhibitor streptomycin, although binding in the same pocket as gentamicin, is orientated such that no potential phosphorylation sites are adjacent to the catalytic aspartate residue. The binding of gentamicin and streptomycin provides structural insights into the substrate selectivity of the APH(2'') subfamily of aminoglycoside phosphotransferases, specifically, the selectivity between the 4,6-disubstituted and the 4,5-disubstituted aminoglycosides.
Figures
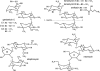
FIG. 1.
Structures of gentamicin, kanamycin, streptomycin, and neomycin. Gentamicin and kanamycin are classified as 4,6-disubstituted aminoglycosides, whereas neomycin is an example of a 4,5-disubstituted compound. The three structural variants which comprise gentamicin C are indicated. Amikacin is similar to kanamycin, although the substituent on the N1 amine is a 4-amino-2-hydroxy-1-oxobutyl group. Taken together, the A and B rings of aminoglycosides, such as gentamicin, kanamycin, and neomycin, are commonly known as the neamine moiety.
FIG. 2.
Crystal structure of APH(2′′)-IIa. (a) Stereo view of the APH(2′′)-IIa monomer showing the location of the substrate binding site, indicated by a gentamicin molecule in yellow, and the nucleotide binding site, indicated by a partially transparent AMP molecule (upper panel). The N-terminal domain is at the top of the figure (pale green), with the core subdomain in the center (bright green) and the helical subdomain at the bottom (dark green). The numbering of the secondary structure elements is indicated. (b) Stereo view of the superposition of the gentamicin-APH(2′′)-IIa complex (green ribbons) and the kanamycin-APH(3′)-IIa complex (red ribbons) based upon residues from the core subdomain. The similarity of the N domain and the core subdomain can be seen, along with the differences in the helical subdomain. The location of the substrate binding site is indicated by a yellow gentamicin molecule and a kanamycin molecule in thinner white sticks. (c) Stereo view of the close-up view of the area inside the black box in panel b, showing the substrate binding site with the “top” and the “base” of the binding cleft indicated. The three rings of the gentamicin (yellow sticks) are labeled in boldface type (C-B-A). The kanamycin molecule as it is bound to APH(3′)-IIa is shown as thin white sticks with the three rings labeled in italics (_A_-_B_-C).
FIG. 3.
Gentamicin binding site. (a) Stereo view of gentamicin in the binary gentamicin-APH(2′′)-IIa complex showing composite omit 2_Fo_-Fc electron density (pink; 1.0 σ) for the gentamicin substrate (yellow ball-and-stick representation). The rings are labeled in boldface type (C-B-A). The hydrogen bonding interactions with the substrate are indicated as dashed lines. The kinked helix α9 is at the lower right. The final 2_Fo_-Fc density (blue; 1.0 σ) is shown for some of the protein residues. (b) View in panel a rotated vertically by 90° and showing the gentamicin bound in the refined position (yellow ball-and-stick representation) and in an alternative “upside-down” orientation (thinner gray sticks). The rings are labeled in boldface type for the correct orientation (C-B-A), and for the alternative orientation, only the A and C rings are labeled in gray italics for clarity.
FIG. 4.
Binding sites in the ternary complex. (a) Stereo view of streptomycin in the ternary AMPPCP-streptomycin-APH(2′′)-IIa complex showing composite omit 2_Fo_-Fc density (pink; 1.0 σ) for the streptomycin inhibitor from molecule A (cyan ball-and-stick representation). The equivalent streptomycin conformation from molecule B is overlaid as thin bonds (gray). The location of helices α9 and α10 from the binary gentamicin-APH(2′′)-IIa complex are shown as semitransparent gray coils. (b) Stereo view of the AMPPCP (gray ball-and-stick representation) in the ternary AMPPCP-streptomycin-APH(2′′)-IIa complex showing composite omit 2_Fo_-Fc density (pink; 1.0σ). The magnesium ion is shown as a yellow sphere surrounded by the chelating AMPPCP triphosphate moiety. The hydrogen-bonding interactions with the AMPPCP are indicated by dashed black lines. Final 2_Fo_-Fc densities (blue; 1.0 σ) for surrounding protein residues are shown, along with the 2_Fo_-Fc density for the interdomain linker (gold bonds, left).
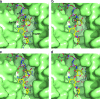
FIG. 5.
Surface representation of the substrate binding site in APH(2′′)-IIa (green) showing the bound gentamicin (yellow sticks) and an AMPPNP molecule (colored sticks; top) from APH(3′)-IIIa modeled into the structure based upon the superposition of the catalytic aspartate loop [residues 189 to 197 in APH(2′′)-IIa; 187 to 195 in APH(3′)-IIIa]. (a) The kanamycin from APH(3′)-IIa is shown as thin gray bonds. APH(3′)-IIa was superimposed on APH(2′′)-IIa based upon the catalytic aspartate loop. The location of this residue (Asp192) is indicated. When the structures are superimposed in this way, the hydroxyl group which is phosphorylated on both substrates is in essentially the same position (indicated by the black circle). (b) The kanamycin molecule modeled in the same conformation as gentamicin. In this orientation, the 2′′-OH of the kanamycin overlaps the equivalent atom on gentamicin (black circle). (c) The superimposed streptomycin from molecule B of the ternary AMPPCP-streptomycin-APH(2′′)-IIa complex, shown as thin gray sticks. The streptomycin occupies approximately the same location as the gentamicin. One of the guanidinium groups on streptomycin A ring projects upwards toward the nucleotide binding site and would clearly interfere with the β- and γ-phosphate groups of an extended ATP triphosphate. (d) A neomycin molecule modeled in the gentamicin binding site such that the A and B rings occupy approximately the same position as the corresponding rings in gentamicin. The C projects toward the helical subdomain, and in this orientation, the D ring of neomycin is hidden behind the helical subdomain.
Similar articles
- Structural basis for the diversity of the mechanism of nucleotide hydrolysis by the aminoglycoside-2''-phosphotransferases.
Smith CA, Toth M, Stewart NK, Maltz L, Vakulenko SB. Smith CA, et al. Acta Crystallogr D Struct Biol. 2019 Dec 1;75(Pt 12):1129-1137. doi: 10.1107/S2059798319015079. Epub 2019 Nov 29. Acta Crystallogr D Struct Biol. 2019. PMID: 31793906 Free PMC article. - Novel aminoglycoside 2''-phosphotransferase identified in a gram-negative pathogen.
Toth M, Frase H, Antunes NT, Vakulenko SB. Toth M, et al. Antimicrob Agents Chemother. 2013 Jan;57(1):452-7. doi: 10.1128/AAC.02049-12. Epub 2012 Nov 5. Antimicrob Agents Chemother. 2013. PMID: 23129050 Free PMC article. - Aminoglycoside resistance mediated by the bifunctional enzyme 6'-N-aminoglycoside acetyltransferase-2"-O-aminoglycoside phosphotransferase.
Culebras E, Martínez JL. Culebras E, et al. Front Biosci. 1999 Jan 1;4:D1-8. doi: 10.2741/culebras. Front Biosci. 1999. PMID: 9872730 Review. - Developing a snapshot of the ATP binding domain(s) of aminoglycoside phosphotransferases.
Perlin MH, Brown SA, Dholakia JN. Perlin MH, et al. Front Biosci. 1999 Jan 1;4:D63-71. doi: 10.2741/perlin. Front Biosci. 1999. PMID: 9872732 Review.
Cited by
- Revisiting the nucleotide and aminoglycoside substrate specificity of the bifunctional aminoglycoside acetyltransferase(6')-Ie/aminoglycoside phosphotransferase(2'')-Ia enzyme.
Frase H, Toth M, Vakulenko SB. Frase H, et al. J Biol Chem. 2012 Dec 21;287(52):43262-9. doi: 10.1074/jbc.M112.416453. Epub 2012 Oct 31. J Biol Chem. 2012. PMID: 23115238 Free PMC article. - In silico analysis of sequenced strains of Clostridium difficile reveals a related set of conjugative transposons carrying a variety of accessory genes.
Brouwer MS, Roberts AP, Mullany P, Allan E. Brouwer MS, et al. Mob Genet Elements. 2012 Jan 1;2(1):8-12. doi: 10.4161/mge.19297. Mob Genet Elements. 2012. PMID: 22754747 Free PMC article. - Whole-genome sequencing of gentamicin-resistant Campylobacter coli isolated from U.S. retail meats reveals novel plasmid-mediated aminoglycoside resistance genes.
Chen Y, Mukherjee S, Hoffmann M, Kotewicz ML, Young S, Abbott J, Luo Y, Davidson MK, Allard M, McDermott P, Zhao S. Chen Y, et al. Antimicrob Agents Chemother. 2013 Nov;57(11):5398-405. doi: 10.1128/AAC.00669-13. Epub 2013 Aug 19. Antimicrob Agents Chemother. 2013. PMID: 23959310 Free PMC article. - Crystal structures of two aminoglycoside kinases bound with a eukaryotic protein kinase inhibitor.
Fong DH, Xiong B, Hwang J, Berghuis AM. Fong DH, et al. PLoS One. 2011 May 9;6(5):e19589. doi: 10.1371/journal.pone.0019589. PLoS One. 2011. PMID: 21573013 Free PMC article. - Small-angle X-ray scattering analysis of the bifunctional antibiotic resistance enzyme aminoglycoside (6') acetyltransferase-ie/aminoglycoside (2'') phosphotransferase-ia reveals a rigid solution structure.
Caldwell SJ, Berghuis AM. Caldwell SJ, et al. Antimicrob Agents Chemother. 2012 Apr;56(4):1899-906. doi: 10.1128/AAC.06378-11. Epub 2012 Jan 30. Antimicrob Agents Chemother. 2012. PMID: 22290965 Free PMC article.
References
- Aimes, R. T., W. Hemmer, and S. S. Taylor. 2000. Serine-53 at the tip of the glycine-rich loop of cAMP-dependent protein kinase: role in catalysis, P-site specificity, and interaction with inhibitors. Biochemistry 398325-8332. - PubMed
- Azucena, E., and S. Mobashery. 2001. Aminoglycoside-modifying enzymes: mechanisms of catalytic processes and inhibition. Drug Resist. Updates 4106-117. - PubMed
- Burk, D. L., and A. M. Berghuis. 2002. Protein kinase inhibitors and antibiotic resistance. Pharmacol. Ther. 93283-292. - PubMed
- Burk, D. L., W. C. Hon, A. K. Leung, and A. M. Berghuis. 2001. Structural analysis of nucleotide binding to an aminoglycoside phosphotransferase. Biochemistry 408756-8764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources