From the Cover: Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering - PubMed (original) (raw)
From the Cover: Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering
Juan Huang et al. Proc Natl Acad Sci U S A. 2009.
Abstract
With the completion of genome sequences of major model organisms, increasingly sophisticated genetic tools are necessary for investigating the complex and coordinated functions of genes. Here we describe a genetic manipulation system termed "genomic engineering" in Drosophila. Genomic engineering is a 2-step process that combines the ends-out (replacement) gene targeting with phage integrase phiC31-mediated DNA integration. First, through an improved and modified gene targeting method, a founder knock-out line is generated by deleting the target gene and replacing it with an integration site of phiC31. Second, DNA integration by phiC31 is used to reintroduce modified target-gene DNA into the native locus in the founder knock-out line. Genomic engineering permits directed and highly efficient modifications of a chosen genomic locus into virtually any desired mutant allele. We have successfully applied the genomic engineering scheme on 6 different genes and have generated at their loci more than 70 unique alleles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Genomic engineering by targeted site-specific DNA integration. (A) A modified ends-out gene-targeting approach is used to delete the target gene first. The targeting donor DNA fragment contains 5′ and 3′ homologous arms (“arm”) flanking the target-gene locus, a loxP-flanked _white_+ (w+) transgenic marker juxtaposed by an attP site of φC31. (B) In the knock-out mutant (“founder knock-out line” or founder line), the target gene is effectively replaced by the loxP-flanked _w_+ marker juxtaposed by a single φC31 attP site. (C) The _w_+ marker is removed by Cre recombinase in the founder line, leaving only the attP and loxP at the deletion locus. (D) The deleted genomic DNA of the target gene is engineered in vitro to incorporate desired modifications (“*target gene*”) on an integration vector (pGE-attB) that carries an attB site together with a _w_+ marker. It will then be integrated into the deletion locus of the founder line through φC31-mediated DNA integration. (E) The resulted “integration mutant allele” has the engineered target gene restored (with modifications) at its original genomic locus together with _w_+ and vector sequences (“_AmpR_”). (F) Extra vector sequences, together with _w_+ can be removed by Cre recombinase, to generate a final engineered-mutant allele containing solely the engineered target gene flanked by attR and loxP sites.
Fig. 2.
Quantification of the DE-Cad expression levels in wild type, DE-Cad(rescue), and DE-Cad::GFP. (A) A sample Western blot showing the DE-Cad expression levels in wild type, DE-Cad::GFP (see Fig. 2 A and B), and DE-Cad(rescue) homozygous embryos. Embryos were in mixed stages (24-h collection under 25 °C). (Top) Because the majority of DE-Cad proteins undergoes internal cleavage (25), the rat anti-DE-Cad monoclonal antibody (DCAD2) (25) recognizes a single 150kDa band in all 3 samples. (Middle) To confirm the identity of DE-Cad::GFP, we also blotted the same samples with anti-GFP antibody. Only in DE-Cad::GFP sample the antibody recognized a single band around 100 kDa, which corresponds to the carboxyl-terminal half of cleaved DE-Cad::GFP (26). (Bottom) α-tublulin was used as loading controls. (B) Quantitative measurements of DE-Cad protein levels in wild type, DE-Cad(rescue), and DE-Cad::GFP that were based on multiple Western blot results (DE-Cad(rescue): n = 3; DE-Cad::GFP: n = 4).
Fig. 3.
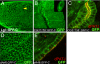
Fluorescent knock-in alleles of DE-Cadherin and crumbs. (A) Protein domain structures of DE-Cad, Crb, and their fluorescent knock-in alleles. In all DE-Cad knock-in alleles, the fluorescent proteins were fused to the C terminus. In 3 Crb::GFP alleles, the GFP was inserted at 2,121 aa (Crb:GFP-A), 2,156 aa (Crb::GFP-B), and 2,189 aa (Crb::GFP-C), respectively. Note that not all of the 30 EGF repeats of Crb are drawn. (B–E) Subcellular localization patterns of DE-Cad::GFP, DE-Cad::PAGFP (photoactivatable GFP), DE-Cad::mTomato, DE-Cad::mCherry in live pupal (B, D, and E) or late embryonic (C) epithelia. All alleles rescued DE-Cad founder lines and were homozygous-viable, but only DE-Cad::GFP and DE-Cad::PAGFP showed clean localization at the adherens junction (B and C). Note the intracellular aggregates of DE-Cad::mTomato and DE-Cad::mCherry in (D) and (E). In (C) the yellow boxes highlight the region before (Top) and after (Bottom) the UV laser irradiation in the same sample. DE-Cad::PAGFP is only fluorescent after UV irradiation. DE-Cad::GFP knock-in homozygotes provide a clean and homogenous background of DE-Cad::GFP, whose expression level is virtually identical to the DE-Cad in wild type (see Fig. 2). (F–H) Subcellular localization of Crb::GFP-A, Crb::GFP-B, and Crb::GFP-C in live embryonic epithelia (stage 11). crb::GFP-A and crb::GFP-C complemented crbGX24 and were homozygous viable. They show normal localization along the apical-lateral boundary of the epithelial cells (F and H). In contrast, Crb::GFP-B shows a disrupted localization pattern (G). crb::GFP-B failed to complement crbGX24 and crb11A22, and is homozygous lethal. All images were taken as the tangential view of the epithelia.
Fig. 4.
Tissue and subcellular localization patterns of Lgl::GFP, CG31158::GFP, and dArf6::GFP knock-in mutants. (A) Subcellular localization of Lgl::GFP-C in live stage 11 embryonic epithelial cell. Lgl::GPF-C shows localization along the basolateral cortex in postmitotic cells, but is diffused in mitotic cells (one of them highlighted by the yellow arrowhead), consistent with previous reports based on Lgl antibodies (27). (B–E) Because both CG31158::GFP and dArf6::GFP-C are too weak to be directly detectable in live embryos by confocal microscopy, embryos are immunostained with anti-GFP antibody. (B) In this tangential-section view of embryonic epithelial cells, CG31158::GFP-C is cytoplasmic but predominantly cortical. It also shows strong expression in CNS in late stage embryos (Inset). (C) The subcellular localization of CG31158. In this cross-section view of embryonic gut epithelial cells, the apical polarity protein dPATJ (red) is seen exclusively at the apical side facing the lumen, while CG31158::GFP-C (green) localizes all around cell cortex. (D) Immunofluorescence by anti-GFP antibody visualizes dArf6::GFP-C has a punctuated pattern along the cell cortex or membrane in this tangential-section view of embryonic epithelial cells. dArf6::GFP-C does not show strong CNS expression in later embryos. (E) Unlike CG31158::GFP-C, dArf6::GFP-C (green) does not overlap extensively with dPATJ (red) in this cross-section view of embryonic epithelial cells.
Similar articles
- Successive and targeted DNA integrations in the Drosophila genome by Bxb1 and phiC31 integrases.
Huang J, Ghosh P, Hatfull GF, Hong Y. Huang J, et al. Genetics. 2011 Sep;189(1):391-5. doi: 10.1534/genetics.111.129247. Epub 2011 Jun 6. Genetics. 2011. PMID: 21652525 Free PMC article. - Targeted engineering of the Drosophila genome.
Huang J, Zhou W, Dong W, Hong Y. Huang J, et al. Fly (Austin). 2009 Oct-Dec;3(4):274-7. doi: 10.4161/fly.9978. Epub 2009 Oct 1. Fly (Austin). 2009. PMID: 19823033 - Robust ΦC31-Mediated Genome Engineering in Drosophila melanogaster Using Minimal attP/attB Phage Sites.
Voutev R, Mann RS. Voutev R, et al. G3 (Bethesda). 2018 May 4;8(5):1399-1402. doi: 10.1534/g3.118.200051. G3 (Bethesda). 2018. PMID: 29523637 Free PMC article. - Genome-wide manipulations of Drosophila melanogaster with transposons, Flp recombinase, and ΦC31 integrase.
Venken KJ, Bellen HJ. Venken KJ, et al. Methods Mol Biol. 2012;859:203-28. doi: 10.1007/978-1-61779-603-6_12. Methods Mol Biol. 2012. PMID: 22367874 Review. - Genome engineering: Drosophila melanogaster and beyond.
Venken KJ, Sarrion-Perdigones A, Vandeventer PJ, Abel NS, Christiansen AE, Hoffman KL. Venken KJ, et al. Wiley Interdiscip Rev Dev Biol. 2016 Mar-Apr;5(2):233-67. doi: 10.1002/wdev.214. Epub 2015 Oct 8. Wiley Interdiscip Rev Dev Biol. 2016. PMID: 26447401 Free PMC article. Review.
Cited by
- Intracellular trafficking of Notch orchestrates temporal dynamics of Notch activity in the fly brain.
Wang M, Han X, Liu C, Takayama R, Yasugi T, Ei SI, Nagayama M, Tanaka Y, Sato M. Wang M, et al. Nat Commun. 2021 Apr 7;12(1):2083. doi: 10.1038/s41467-021-22442-3. Nat Commun. 2021. PMID: 33828096 Free PMC article. - The PCP pathway regulates Baz planar distribution in epithelial cells.
Aigouy B, Le Bivic A. Aigouy B, et al. Sci Rep. 2016 Sep 14;6:33420. doi: 10.1038/srep33420. Sci Rep. 2016. PMID: 27624969 Free PMC article. - Vascular control of the Drosophila haematopoietic microenvironment by Slit/Robo signalling.
Morin-Poulard I, Sharma A, Louradour I, Vanzo N, Vincent A, Crozatier M. Morin-Poulard I, et al. Nat Commun. 2016 May 19;7:11634. doi: 10.1038/ncomms11634. Nat Commun. 2016. PMID: 27193394 Free PMC article. - Polychaetoid/ZO-1 strengthens cell junctions under tension while localizing differently than core adherens junction proteins.
Schmidt A, Finegan T, Häring M, Kong D, Fletcher AG, Alam Z, Grosshans J, Wolf F, Peifer M. Schmidt A, et al. bioRxiv [Preprint]. 2023 Mar 1:2023.03.01.530634. doi: 10.1101/2023.03.01.530634. bioRxiv. 2023. PMID: 36909597 Free PMC article. Updated. Preprint. - Conserved function of the matriptase-prostasin proteolytic cascade during epithelial morphogenesis.
Drees L, Königsmann T, Jaspers MHJ, Pflanz R, Riedel D, Schuh R. Drees L, et al. PLoS Genet. 2019 Jan 2;15(1):e1007882. doi: 10.1371/journal.pgen.1007882. eCollection 2019 Jan. PLoS Genet. 2019. PMID: 30601807 Free PMC article.
References
- Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila. Science. 2000;288:2013–2018. - PubMed
- Venken KJ, Bellen HJ. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat Rev Genet. 2005;6:167–178. - PubMed
- Adams MD, Sekelsky JJ. From sequence to phenotype: reverse genetics in Drosophila melanogaster. Nat Rev Genet. 2002;3:189–198. - PubMed
- Margolis B, Borg JP. Apicobasal polarity complexes. J Cell Sci. 2005;118:5157–5159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases