Mitochondrial accumulation of polyubiquitinated proteins and differential regulation of apoptosis by polyubiquitination sites Lys-48 and -63 - PubMed (original) (raw)
Mitochondrial accumulation of polyubiquitinated proteins and differential regulation of apoptosis by polyubiquitination sites Lys-48 and -63
Faneng Sun et al. J Cell Mol Med. 2009 Aug.
Abstract
Proteins tagged with lysine (Lys, K) 48 polyubiquitins chains are destined for degradation by the 26S proteasomal system. Impairment of the ubiquitin proteasome system (UPS) function culminates in the accumulation of polyubiquitinated proteins in many neurodegenerative conditions including Parkinson's disease (PD). Nevertheless, the cellular mechanisms underlying cell death induced by an impaired UPS are still not clear. Intriguingly, recent studies indicate that several proteins associated with familial PD are capable of promoting the assembly of Lys-63 polyubiquitin chains. Therefore, the objective of this study was to examine the role of K48 and K63 ubiquitination in mitochondria-mediated apoptosis in in vitro models of dopaminergic degeneration. Exposure of the widely used proteasome inhibitor MG-132 to dopaminergic neuronal cell line (N27) induced a rapid accumulation of polyubiquitinated proteins in the mitochondria. This appears to result in the preferential association of ubiquitin conjugates in the outer membrane and polyubiquitination of outer membrane proteins. Interestingly, the ubiquitin(K48R) mutant effectively rescued cells from MG-132-induced mitochondrial apoptosis without altering the antioxidant status of cells; whereas the ubiquitin(K63R) mutant augmented the proapoptotic effect of MG-132. Herein, we report a novel conclusion that polyubiquitinated proteins, otherwise subjected to proteasomal degradation, preferentially accumulate in the mitochondria during proteolytic stress; and that polyubiquitination of Lys-48 and Lys-63 are key determinants of mitochondria-mediated cell death during proteasomal dysfunction. Together, these findings yield novel insights into a crosstalk between the UPS and mitochondria in dopaminergic neuronal cells.
Figures
Figure 1
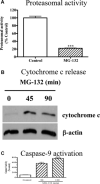
(A) Activation of mitochondrial apoptosis by MG‐132. N27 cells were exposed to 2.5 μM MG‐132 for 5 min. and then chymotrypsin‐like proteasomal activity was assayed as described in the methods. The data represent mean ± S.E.M., _n_= 6, ***P < 0.001. (B) Cytochrome c release. N27 cells were treated with 2.5 μM MG‐132 for 45 or 90 min., and the level of cytosolic cytochrome c was examined by Western blot using cytochrome c antibody. The membranes were reprobed with β‐actin antibody. (C) Caspase‐9 activation. Caspase‐9 activity was assayed in the cells exposed to MG‐132 for 90 or 120 min. as described in the methods section. The activity was expressed as the percentage of vehicle‐treated cells. _n_= 6, ***P < 0.001 as compared to control group.
Figure 2
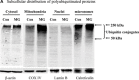
Mitochondrial accumulation of ubiquitinated proteins. (A) Subcellular distribution of polyubiquitin proteins. N27 cells were treated with 2.5 μM MG‐132 for 40 min. The cytosolic, mitochondrial, nuclear and microsomal fractions were prepared as described in the methods. Equal amounts of proteins were used for Western blot analysis using antibodies against ubiquitin, and subcellular markers β‐actin (cytosol), COX IV (mitochondria), lamin B (Nuclei) and calreticulin (microsome). (B) Time‐dependent elevation of polyubiquitinated proteins in mitochondria. Cytosolic and mitochondrial fractions were prepared from N27 cells exposed to 2.5 μM MG‐132 for 20, 40 or 60 min., and processed for ubiquitin immunoblotting. (C) Verification of mitochondria ubiquitination by sucrose gradient. Crude mitochondria isolated from N27 cells exposed to MG‐132 (2.5 μM for 40 min.) were subjected to sucrose gradient separation as described in the methods section. All the fractions collected were resolved on SDS‐PAGE and blotted with ubiquitin, β‐actin and/or COX IV antibodies. (D) Dissociation of ubiquitin conjugates from mitochondrial outer membrane. Mitochondria isolated from vehicle (Con) or MG‐132‐treated cells (MG) were incubated with a high pH buffer as described in the methods section, and then mitochondria were spun down. Both the mitochondrial pellets (P) and the equal proportion of the supernatant (S) were separated on SDS‐PAGE and immunoblotted with antibodies for ubiquitin, VDAC and COX IV. (E) Ubiquitination of mitochondrial proteins in vitro. The reaction was carried out by incubating the mitochondrial suspension (4 mg/ml) with ubiquitination enzymes (9.6 μg for fraction A and B included in the kits), ubiquitin (8 μg), ubiquitin aldehyde and an energy source. Mitochondria were then recovered from the reaction mixture for Western blotting using ubiquitin or cytochrome c antibodies.
Figure 2
Mitochondrial accumulation of ubiquitinated proteins. (A) Subcellular distribution of polyubiquitin proteins. N27 cells were treated with 2.5 μM MG‐132 for 40 min. The cytosolic, mitochondrial, nuclear and microsomal fractions were prepared as described in the methods. Equal amounts of proteins were used for Western blot analysis using antibodies against ubiquitin, and subcellular markers β‐actin (cytosol), COX IV (mitochondria), lamin B (Nuclei) and calreticulin (microsome). (B) Time‐dependent elevation of polyubiquitinated proteins in mitochondria. Cytosolic and mitochondrial fractions were prepared from N27 cells exposed to 2.5 μM MG‐132 for 20, 40 or 60 min., and processed for ubiquitin immunoblotting. (C) Verification of mitochondria ubiquitination by sucrose gradient. Crude mitochondria isolated from N27 cells exposed to MG‐132 (2.5 μM for 40 min.) were subjected to sucrose gradient separation as described in the methods section. All the fractions collected were resolved on SDS‐PAGE and blotted with ubiquitin, β‐actin and/or COX IV antibodies. (D) Dissociation of ubiquitin conjugates from mitochondrial outer membrane. Mitochondria isolated from vehicle (Con) or MG‐132‐treated cells (MG) were incubated with a high pH buffer as described in the methods section, and then mitochondria were spun down. Both the mitochondrial pellets (P) and the equal proportion of the supernatant (S) were separated on SDS‐PAGE and immunoblotted with antibodies for ubiquitin, VDAC and COX IV. (E) Ubiquitination of mitochondrial proteins in vitro. The reaction was carried out by incubating the mitochondrial suspension (4 mg/ml) with ubiquitination enzymes (9.6 μg for fraction A and B included in the kits), ubiquitin (8 μg), ubiquitin aldehyde and an energy source. Mitochondria were then recovered from the reaction mixture for Western blotting using ubiquitin or cytochrome c antibodies.
Figure 2
Mitochondrial accumulation of ubiquitinated proteins. (A) Subcellular distribution of polyubiquitin proteins. N27 cells were treated with 2.5 μM MG‐132 for 40 min. The cytosolic, mitochondrial, nuclear and microsomal fractions were prepared as described in the methods. Equal amounts of proteins were used for Western blot analysis using antibodies against ubiquitin, and subcellular markers β‐actin (cytosol), COX IV (mitochondria), lamin B (Nuclei) and calreticulin (microsome). (B) Time‐dependent elevation of polyubiquitinated proteins in mitochondria. Cytosolic and mitochondrial fractions were prepared from N27 cells exposed to 2.5 μM MG‐132 for 20, 40 or 60 min., and processed for ubiquitin immunoblotting. (C) Verification of mitochondria ubiquitination by sucrose gradient. Crude mitochondria isolated from N27 cells exposed to MG‐132 (2.5 μM for 40 min.) were subjected to sucrose gradient separation as described in the methods section. All the fractions collected were resolved on SDS‐PAGE and blotted with ubiquitin, β‐actin and/or COX IV antibodies. (D) Dissociation of ubiquitin conjugates from mitochondrial outer membrane. Mitochondria isolated from vehicle (Con) or MG‐132‐treated cells (MG) were incubated with a high pH buffer as described in the methods section, and then mitochondria were spun down. Both the mitochondrial pellets (P) and the equal proportion of the supernatant (S) were separated on SDS‐PAGE and immunoblotted with antibodies for ubiquitin, VDAC and COX IV. (E) Ubiquitination of mitochondrial proteins in vitro. The reaction was carried out by incubating the mitochondrial suspension (4 mg/ml) with ubiquitination enzymes (9.6 μg for fraction A and B included in the kits), ubiquitin (8 μg), ubiquitin aldehyde and an energy source. Mitochondria were then recovered from the reaction mixture for Western blotting using ubiquitin or cytochrome c antibodies.
Figure 2
Mitochondrial accumulation of ubiquitinated proteins. (A) Subcellular distribution of polyubiquitin proteins. N27 cells were treated with 2.5 μM MG‐132 for 40 min. The cytosolic, mitochondrial, nuclear and microsomal fractions were prepared as described in the methods. Equal amounts of proteins were used for Western blot analysis using antibodies against ubiquitin, and subcellular markers β‐actin (cytosol), COX IV (mitochondria), lamin B (Nuclei) and calreticulin (microsome). (B) Time‐dependent elevation of polyubiquitinated proteins in mitochondria. Cytosolic and mitochondrial fractions were prepared from N27 cells exposed to 2.5 μM MG‐132 for 20, 40 or 60 min., and processed for ubiquitin immunoblotting. (C) Verification of mitochondria ubiquitination by sucrose gradient. Crude mitochondria isolated from N27 cells exposed to MG‐132 (2.5 μM for 40 min.) were subjected to sucrose gradient separation as described in the methods section. All the fractions collected were resolved on SDS‐PAGE and blotted with ubiquitin, β‐actin and/or COX IV antibodies. (D) Dissociation of ubiquitin conjugates from mitochondrial outer membrane. Mitochondria isolated from vehicle (Con) or MG‐132‐treated cells (MG) were incubated with a high pH buffer as described in the methods section, and then mitochondria were spun down. Both the mitochondrial pellets (P) and the equal proportion of the supernatant (S) were separated on SDS‐PAGE and immunoblotted with antibodies for ubiquitin, VDAC and COX IV. (E) Ubiquitination of mitochondrial proteins in vitro. The reaction was carried out by incubating the mitochondrial suspension (4 mg/ml) with ubiquitination enzymes (9.6 μg for fraction A and B included in the kits), ubiquitin (8 μg), ubiquitin aldehyde and an energy source. Mitochondria were then recovered from the reaction mixture for Western blotting using ubiquitin or cytochrome c antibodies.
Figure 2
Mitochondrial accumulation of ubiquitinated proteins. (A) Subcellular distribution of polyubiquitin proteins. N27 cells were treated with 2.5 μM MG‐132 for 40 min. The cytosolic, mitochondrial, nuclear and microsomal fractions were prepared as described in the methods. Equal amounts of proteins were used for Western blot analysis using antibodies against ubiquitin, and subcellular markers β‐actin (cytosol), COX IV (mitochondria), lamin B (Nuclei) and calreticulin (microsome). (B) Time‐dependent elevation of polyubiquitinated proteins in mitochondria. Cytosolic and mitochondrial fractions were prepared from N27 cells exposed to 2.5 μM MG‐132 for 20, 40 or 60 min., and processed for ubiquitin immunoblotting. (C) Verification of mitochondria ubiquitination by sucrose gradient. Crude mitochondria isolated from N27 cells exposed to MG‐132 (2.5 μM for 40 min.) were subjected to sucrose gradient separation as described in the methods section. All the fractions collected were resolved on SDS‐PAGE and blotted with ubiquitin, β‐actin and/or COX IV antibodies. (D) Dissociation of ubiquitin conjugates from mitochondrial outer membrane. Mitochondria isolated from vehicle (Con) or MG‐132‐treated cells (MG) were incubated with a high pH buffer as described in the methods section, and then mitochondria were spun down. Both the mitochondrial pellets (P) and the equal proportion of the supernatant (S) were separated on SDS‐PAGE and immunoblotted with antibodies for ubiquitin, VDAC and COX IV. (E) Ubiquitination of mitochondrial proteins in vitro. The reaction was carried out by incubating the mitochondrial suspension (4 mg/ml) with ubiquitination enzymes (9.6 μg for fraction A and B included in the kits), ubiquitin (8 μg), ubiquitin aldehyde and an energy source. Mitochondria were then recovered from the reaction mixture for Western blotting using ubiquitin or cytochrome c antibodies.
Figure 3
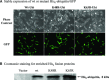
Effect of wt or mutant ubiquitin on cellular Redox status. (A) Stable expression of wt or mutant His6‐ubiquitin/GFP. Phase contrast and GFP images for cells stably expressing the linear fusion of wt, K48R or K63R ubiquitin/GFP. (B) Coomassie staining for enriched His6 fusion proteins. His6‐tagged proteins were enriched from three lines of stable cells using Ni‐IMAC resin, and resolved on SDS‐PAGE and then processed for Coomassie blue staining. The arrow indicates the protein of about 8 kD expressed in the cells, and the size roughly matches the molecular weight of His6‐ubiquitin. (C) Mitochondrial superoxide. Cells stably expressing His6‐tagged ubiquitin/GFP were treated with either 2.5 μM MG‐132 or 1 μM rotenone (positive control) for 1 hr and then incubated with the MitoSOX for 15 min. The live images were then analysed with confocal microscopy. (D) Cellular glutathione levels. Cells were treated with 2.5 μM MG‐132 for 1 hr. Cellular glutathione levels were determined as described in the methods. Data represent results of two experiments performed in triplicate.
Figure 3
Effect of wt or mutant ubiquitin on cellular Redox status. (A) Stable expression of wt or mutant His6‐ubiquitin/GFP. Phase contrast and GFP images for cells stably expressing the linear fusion of wt, K48R or K63R ubiquitin/GFP. (B) Coomassie staining for enriched His6 fusion proteins. His6‐tagged proteins were enriched from three lines of stable cells using Ni‐IMAC resin, and resolved on SDS‐PAGE and then processed for Coomassie blue staining. The arrow indicates the protein of about 8 kD expressed in the cells, and the size roughly matches the molecular weight of His6‐ubiquitin. (C) Mitochondrial superoxide. Cells stably expressing His6‐tagged ubiquitin/GFP were treated with either 2.5 μM MG‐132 or 1 μM rotenone (positive control) for 1 hr and then incubated with the MitoSOX for 15 min. The live images were then analysed with confocal microscopy. (D) Cellular glutathione levels. Cells were treated with 2.5 μM MG‐132 for 1 hr. Cellular glutathione levels were determined as described in the methods. Data represent results of two experiments performed in triplicate.
Figure 3
Effect of wt or mutant ubiquitin on cellular Redox status. (A) Stable expression of wt or mutant His6‐ubiquitin/GFP. Phase contrast and GFP images for cells stably expressing the linear fusion of wt, K48R or K63R ubiquitin/GFP. (B) Coomassie staining for enriched His6 fusion proteins. His6‐tagged proteins were enriched from three lines of stable cells using Ni‐IMAC resin, and resolved on SDS‐PAGE and then processed for Coomassie blue staining. The arrow indicates the protein of about 8 kD expressed in the cells, and the size roughly matches the molecular weight of His6‐ubiquitin. (C) Mitochondrial superoxide. Cells stably expressing His6‐tagged ubiquitin/GFP were treated with either 2.5 μM MG‐132 or 1 μM rotenone (positive control) for 1 hr and then incubated with the MitoSOX for 15 min. The live images were then analysed with confocal microscopy. (D) Cellular glutathione levels. Cells were treated with 2.5 μM MG‐132 for 1 hr. Cellular glutathione levels were determined as described in the methods. Data represent results of two experiments performed in triplicate.
Figure 4
Effect of mutant ubiquitin on apoptosis. (A) Cytochrome c release. N27 cells stably expressing wt or mutant ubiquitins (K48R or K63R) were treated with 2.5 μM MG‐132 for 45 min., and the cytosolic fractions were subjected to Western blot analysis using cytochrome c. The membranes were reprobed with β‐actin and COX 4 antibodies. (B) Caspase‐9 activity, (C) Caspase‐3 activity and (D) DNA fragmentation. N27 cells stably expressing wt or mutant ubiquitins (K48R or K63R) were treated with MG‐132 for 120 min. Enzymatic activities of caspase‐9 and ‐3 and DNA fragmentation were determined as described previously. Data for caspase‐9 represent results of two separate experiments with a total of 11 individual samples, respectively; the data for caspase‐3 were derived from three experiments with a total of 16 individual samples, respectively. Two separate experiments with a total of six separate samples were analysed for DNA fragmentation. Values were expressed as the percentage of vehicle‐treated wt ubiquitin control group. **P < 0.01, ***P < 0.001 compared with individual control groups; ##P < 0.01, ###P < 0.001, comparison between indicated groups.
Similar articles
- Proteasome inhibitor-induced apoptosis is mediated by positive feedback amplification of PKCdelta proteolytic activation and mitochondrial translocation.
Sun F, Kanthasamy A, Song C, Yang Y, Anantharam V, Kanthasamy AG. Sun F, et al. J Cell Mol Med. 2008 Dec;12(6A):2467-81. doi: 10.1111/j.1582-4934.2008.00293.x. Epub 2008 Feb 24. J Cell Mol Med. 2008. PMID: 18298651 Free PMC article. - Inhibition of proteasome reveals basal mitochondrial ubiquitination.
Sulkshane P, Duek I, Ram J, Thakur A, Reis N, Ziv T, Glickman MH. Sulkshane P, et al. J Proteomics. 2020 Oct 30;229:103949. doi: 10.1016/j.jprot.2020.103949. Epub 2020 Aug 31. J Proteomics. 2020. PMID: 32882436 - Dual roles for ubiquitination in the processing of sperm organelles after fertilization.
Hajjar C, Sampuda KM, Boyd L. Hajjar C, et al. BMC Dev Biol. 2014 Feb 15;14:6. doi: 10.1186/1471-213X-14-6. BMC Dev Biol. 2014. PMID: 24528894 Free PMC article. - K63-linked ubiquitination and neurodegeneration.
Lim KL, Lim GG. Lim KL, et al. Neurobiol Dis. 2011 Jul;43(1):9-16. doi: 10.1016/j.nbd.2010.08.001. Epub 2010 Aug 7. Neurobiol Dis. 2011. PMID: 20696248 Review. - Proteasome-independent functions of lysine-63 polyubiquitination in plants.
Romero-Barrios N, Vert G. Romero-Barrios N, et al. New Phytol. 2018 Feb;217(3):995-1011. doi: 10.1111/nph.14915. Epub 2017 Nov 30. New Phytol. 2018. PMID: 29194634 Review.
Cited by
- Identification of ubiquitin Ser57 kinases regulating the oxidative stress response in yeast.
Hepowit NL, Pereira KN, Tumolo JM, Chazin WJ, MacGurn JA. Hepowit NL, et al. Elife. 2020 Oct 19;9:e58155. doi: 10.7554/eLife.58155. Elife. 2020. PMID: 33074099 Free PMC article. - Differential ubiquitination and degradation of huntingtin fragments modulated by ubiquitin-protein ligase E3A.
Bhat KP, Yan S, Wang CE, Li S, Li XJ. Bhat KP, et al. Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):5706-11. doi: 10.1073/pnas.1402215111. Epub 2014 Mar 31. Proc Natl Acad Sci U S A. 2014. PMID: 24706802 Free PMC article. - Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy?
Higgins GC, Coughlan MT. Higgins GC, et al. Br J Pharmacol. 2014 Apr;171(8):1917-42. doi: 10.1111/bph.12503. Br J Pharmacol. 2014. PMID: 24720258 Free PMC article. Review. - Aging, Proteotoxicity, Mitochondria, Glycation, NAD and Carnosine: Possible Inter-Relationships and Resolution of the Oxygen Paradox.
Hipkiss AR. Hipkiss AR. Front Aging Neurosci. 2010 Mar 18;2:10. doi: 10.3389/fnagi.2010.00010. eCollection 2010. Front Aging Neurosci. 2010. PMID: 20552048 Free PMC article. - Cross-talk between mitochondria and proteasome in Parkinson's disease pathogenesis.
Branco DM, Arduino DM, Esteves AR, Silva DF, Cardoso SM, Oliveira CR. Branco DM, et al. Front Aging Neurosci. 2010 May 21;2:17. doi: 10.3389/fnagi.2010.00017. eCollection 2010. Front Aging Neurosci. 2010. PMID: 20577640 Free PMC article.
References
- Glickman MH, Ciechanover A. The ubiquitin‐proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002; 82: 373–428. - PubMed
- Sun F, Kanthasamy A, Anantharam V, et al . Environmental neurotoxic chemicals‐induced ubiquitin proteasome system dysfunction in the pathogenesis and progression of Parkinson’s disease. Pharmacol Ther. 2007; 114: 327–44. - PubMed
- Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004; 1695: 55–72. - PubMed
- Abou‐Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006; 7: 207–19. - PubMed
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003; 53: S26–36; discussion S36–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES10586/ES/NIEHS NIH HHS/United States
- NS38644/NS/NINDS NIH HHS/United States
- R01 NS038644-11/NS/NINDS NIH HHS/United States
- R01 NS065167-01/NS/NINDS NIH HHS/United States
- R01 ES010586-08/ES/NIEHS NIH HHS/United States
- R01 NS038644/NS/NINDS NIH HHS/United States
- R01 ES010586/ES/NIEHS NIH HHS/United States
- NS65167/NS/NINDS NIH HHS/United States
- R01 NS065167/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources