Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network - PubMed (original) (raw)
Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network
Robin W Klemm et al. J Cell Biol. 2009.
Abstract
The trans-Golgi network (TGN) is the major sorting station in the secretory pathway of all eukaryotic cells. How the TGN sorts proteins and lipids to generate the enrichment of sphingolipids and sterols at the plasma membrane is poorly understood. To address this fundamental question in membrane trafficking, we devised an immunoisolation procedure for specific recovery of post-Golgi secretory vesicles transporting a transmembrane raft protein from the TGN to the cell surface in the yeast Saccharomyces cerevisiae. Using a novel quantitative shotgun lipidomics approach, we could demonstrate that TGN sorting selectively enriched ergosterol and sphingolipid species in the immunoisolated secretory vesicles. This finding, for the first time, indicates that the TGN exhibits the capacity to sort membrane lipids. Furthermore, the observation that the immunoisolated vesicles exhibited a higher membrane order than the late Golgi membrane, as measured by C-Laurdan spectrophotometry, strongly suggests that lipid rafts play a role in the TGN-sorting machinery.
Figures
Figure 1.
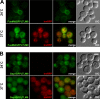
Intracellular accumulation of the immunoisolation baits at the restrictive temperature 37°C in sec6-4 cells. (A) FusMidGFPLTLM9, the raft-carrier cargo immunoisolation bait, and InvRFP expressed for 45 min. At the permissive temperature 24°C, FusMidGFPLTLM9 reached the PM, whereas InvRFP was secreted and therefore not visible (for InvRFP secretion, see also
Fig. S3 B
). At the restrictive temperature 37°C, both proteins accumulated intracellularly. (B) Gap1GFPLTLM9, the TGN/E immunoisolation bait, and InvRFP expressed for 45 min. At the permissive temperature 24°C, InvRFP was secreted and, thus, not visible (Fig. S3 B), and Gap1GFPLTLM9 localized to intracellular compartments. At the restrictive temperature 37°C, both proteins accumulated intracellularly. DIC, differential interference contrast. Bars, 2 µm.
Figure 2.
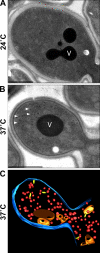
Accumulation of secretory vesicles in sec6-4 cells at the restrictive temperature 37°C. (A) Transmission electron micrograph of a budding sec6-4 cell cultured at the permissive temperature 24°C. (B) Transmission electron micrograph of a budding sec6-4 cell cultured for 45 min at the restrictive temperature 37°C. White arrowheads indicate accumulated secretory vesicles with a diameter of ∼100 nm. (C) 3D reconstruction of a budding cell with accumulated secretory vesicles (red) in a tomogram recorded from a 200-nm thick section prepared from the same culture of cells as shown in B; the PM is reconstructed in blue, the vacuole is orange, and other organelles such as Golgi cisternae or endosomal structures are in yellow. V, vacuole. Bars, 1 µm.
Figure 3.
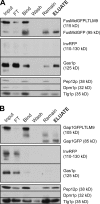
Immunoisolation of FusMidp-vesicles and the TGN/E. (A and B) Several organelle markers were monitored throughout the isolation of FusMidp-vesicles (A) and the TGN/E (B), with GFP detecting the respective baits. InvRFP was used as a specific marker for HDSVs. Gas1p is the major GPI-anchored protein in yeast and is an LDSV marker. Pep12p is the late endosome t-SNARE, Dpm1p is an ER protein, and Tlg1p is a late Golgi marker. Input, sucrose gradient fraction 7 (A) and 4 (B); FT, flow through; Bind, material bound to the immunoadsorbent; Wash, supernatant of the last wash; Remain, material not released from the cellulose fibers after TEV cleavage; ELUATE, eluted material released from the immunoadsorbent after TEV cleavage.
Figure 4.
Transmission electron micrographs of FusMidp-vesicles in different magnifications. (A) Overview of FusMidp-vesicles in an electron micrograph shows the morphological homogeneity of the FusMidp-vesicle population with a diameter of ∼100 nm. These isolated vesicles have the same morphological characteristics as the structures that accumulate in sec6-4 cells at 37°C; see Fig. 2 (B and C). (B) Transmission electron micrograph of a single FusMidp-vesicle. The 100-nm vesicular structure is formed by a membrane whose electron density pattern shows the integrity of the lipid bilayer. (C) A group of FusMidp-vesicles. The lipid bilayer is visible as in B. Bars: (A) 1,000 nm; (B and C) 100 nm.
Figure 5.
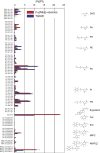
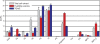
Molecular lipid species composition of FusMidp-vesicles and the donor TGN/E. Quantitative lipidomic analysis of FusMidp-vesicles and the TGN/E allowed absolute quantification of 83 molecular lipid species. Lipid composition is shown in mole percentage to demonstrate the stoichiometric relationship between the lipid species (n = 4 independent experiments; mean estimate ± SD). Chemical structures of membrane lipids are illustrated on the right. Lipid species are annotated by their molecular composition (see Materials and methods). Cer, ceramide; PG, phosphatidylglycerol.
Figure 6.
Lipid class composition of FusMidp-vesicles, the TGN/E, and total cell extract. The mole percentage of lipid class was calculated as the sum of the mole percentage of lipid species of the respective lipid class. (n = 4 independent experiments; mean estimate ± SD). Cer, ceramide; PG, phosphatidylglycerol.
Figure 7.
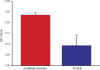
C-Laurdan spectrophotometry reveals higher membrane order for FusMidp-vesicle as compared with the TGN/E. Immunoisolated membranes were stained with C-Laurdan and subsequently analyzed by fluorescence spectrophotometry. The GP is a relative measure for membrane order (see Materials and methods). FusMidp-vesicles (red bar) exhibited a higher GP value than the TGN/E (blue bar; n = 3 independent experiments; mean estimate ± SD).
Similar articles
- Generic sorting of raft lipids into secretory vesicles in yeast.
Surma MA, Klose C, Klemm RW, Ejsing CS, Simons K. Surma MA, et al. Traffic. 2011 Sep;12(9):1139-47. doi: 10.1111/j.1600-0854.2011.01221.x. Epub 2011 Jun 15. Traffic. 2011. PMID: 21575114 - Lipid-dependent protein sorting at the trans-Golgi network.
Surma MA, Klose C, Simons K. Surma MA, et al. Biochim Biophys Acta. 2012 Aug;1821(8):1059-67. doi: 10.1016/j.bbalip.2011.12.008. Epub 2011 Dec 31. Biochim Biophys Acta. 2012. PMID: 22230596 Review. - Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle.
Deng Y, Rivera-Molina FE, Toomre DK, Burd CG. Deng Y, et al. Proc Natl Acad Sci U S A. 2016 Jun 14;113(24):6677-82. doi: 10.1073/pnas.1602875113. Epub 2016 May 31. Proc Natl Acad Sci U S A. 2016. PMID: 27247384 Free PMC article. - Lipid-dependent coupling of secretory cargo sorting and trafficking at the trans-Golgi network.
von Blume J, Hausser A. von Blume J, et al. FEBS Lett. 2019 Sep;593(17):2412-2427. doi: 10.1002/1873-3468.13552. Epub 2019 Jul 30. FEBS Lett. 2019. PMID: 31344259 Free PMC article. Review. - Enrichment of hydroxylated C24- and C26-acyl-chain sphingolipids mediates PIN2 apical sorting at trans-Golgi network subdomains.
Wattelet-Boyer V, Brocard L, Jonsson K, Esnay N, Joubès J, Domergue F, Mongrand S, Raikhel N, Bhalerao RP, Moreau P, Boutté Y. Wattelet-Boyer V, et al. Nat Commun. 2016 Sep 29;7:12788. doi: 10.1038/ncomms12788. Nat Commun. 2016. PMID: 27681606 Free PMC article.
Cited by
- A defect in ATP-citrate lyase links acetyl-CoA production, virulence factor elaboration and virulence in Cryptococcus neoformans.
Griffiths EJ, Hu G, Fries B, Caza M, Wang J, Gsponer J, Gates-Hollingsworth MA, Kozel TR, De Repentigny L, Kronstad JW. Griffiths EJ, et al. Mol Microbiol. 2012 Dec;86(6):1404-23. doi: 10.1111/mmi.12065. Epub 2012 Nov 1. Mol Microbiol. 2012. PMID: 23078142 Free PMC article. - The Sec7 Arf-GEF is recruited to the trans-Golgi network by positive feedback.
Richardson BC, McDonold CM, Fromme JC. Richardson BC, et al. Dev Cell. 2012 Apr 17;22(4):799-810. doi: 10.1016/j.devcel.2012.02.006. Dev Cell. 2012. PMID: 22516198 Free PMC article. - The exomer cargo adaptor structure reveals a novel GTPase-binding domain.
Paczkowski JE, Richardson BC, Strassner AM, Fromme JC. Paczkowski JE, et al. EMBO J. 2012 Nov 5;31(21):4191-203. doi: 10.1038/emboj.2012.268. Epub 2012 Sep 21. EMBO J. 2012. PMID: 23000721 Free PMC article. - Bioanalysis of eukaryotic organelles.
Satori CP, Henderson MM, Krautkramer EA, Kostal V, Distefano MD, Arriaga EA. Satori CP, et al. Chem Rev. 2013 Apr 10;113(4):2733-811. doi: 10.1021/cr300354g. Chem Rev. 2013. PMID: 23570618 Free PMC article. Review. No abstract available. - Palmitoylation regulates the intracellular trafficking and stability of c-Met.
Coleman DT, Gray AL, Kridel SJ, Cardelli JA. Coleman DT, et al. Oncotarget. 2016 May 31;7(22):32664-77. doi: 10.18632/oncotarget.8706. Oncotarget. 2016. PMID: 27081699 Free PMC article.
References
- Aebersold R., Mann M. 2003. Mass spectrometry-based proteomics.Nature. 422:198–207 - PubMed
- Bard F., Malhotra V. 2006. The formation of TGN-to-plasma-membrane transport carriers.Annu. Rev. Cell Dev. Biol. 22:439–455 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous